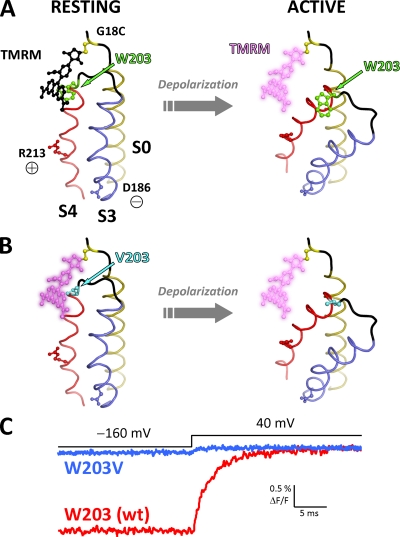
Figure 7.
A proposed relative, activation-dependent motion between S0 and S4. (A; left) A hypothetical model of the BKCa voltage sensor at rest, showing helices S0, S3, and S4 arranged according to the most recent information from disulfide cross-linking efficiency (Liu et al., 2010). S3 (blue) and S4 (red) helices are modeled by homology with the KV1.2-2.1 chimera structure (Protein Data Base accession no. 2R9R) (Long et al., 2007). The two helices are joined by a short helix–loop–helix structure as previously inferred by bimane fluorescence (Semenova et al., 2009). The S0 helix (olive) is modeled as an ideal α helix, whereas its N-terminal flank is shown as a disordered coil (black). A substituted cysteine at position 18 (yellow) is bound by a TMRM molecule (black), which is in close proximity to W203 at the extracellular tip of S4 (green). In this state, TMRM fluorescence is quenched by W203. Voltage-sensing residues D186 (in S3) and R213 (in S4) (Ma et al., 2006) are also shown. (Right) Membrane depolarization induces R213 to move outwards and D186 inwards, causing activation of the voltage sensor domain. This results in a relative motion between the voltage-sensing S3/S4 and S0, increasing the distance between W203 and the fluorophore. As a result, the quenching effect is lifted, producing a large, positive ΔF/F signal. (B) As in A, for channels with mutation W203V. Because of the absence of W203, TMRM is at a bright state when the VSD is at rest and only exhibits a small deflection during voltage sensor activation. (C) Characteristic TMRM fluorescence traces for wt and W203V channels (red and blue, respectively) labeled at position 18. The traces are superimposed at their fluorescence level when the membrane is depolarized. Fluorescence from wt channels at −160 mV (resting VSD state) is quenched because of the intimate association of the fluorophore with W203 (A). In contrast, the fluorophore is not quenched in W203V channels at rest (B) and emits more fluorescence. TMRM fluorescence in both wt and W203V channels increases upon depolarization after a large or small fluorescence deflection, respectively.