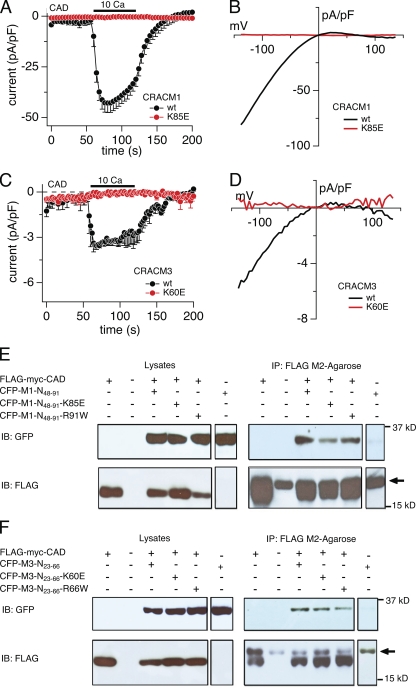
Figure 6.
Lysine residue point mutations of short CRACM1 and CRACM3 N-terminal constructs bind to the CAD of STIM1 but suppress current activation. (A) HEK293 cells were transfected with the FLAG-myc-CAD and CRACM1 or CRACM1-K85E construct, respectively (refer to Materials and methods). Normalized current densities of CAD-mediated CRAC currents at −80 mV are plotted against time of the experiment. Cells were kept in nominal Ca2+-free solution, and the bar indicates the application of external solution containing 10 mM Ca2+. In control experiments, the application of Ca2+ produced an inward current, characteristic of ICRAC (black; n = 7). The K85E mutation completely abolished current activation upon Ca2+ application (red; n = 9). Data represent the average leak-subtracted current densities (pA/pF) evoked by 50-ms voltage ramps from −150 to +150 mV. Error bars indicate SEM. (B and D) Average I-V relationships of CRAC currents extracted at 120 s from representative cells shown in A and C. (C) Cells were transfected with FLAG-myc-CAD and CRACM3 or CRACM3-K60E constructs, respectively, and the average normalized current densities at −80 mV are plotted against time of the experiment. Similar to CRACM1, CRACM3 gave rise to ICRAC when 10 mM Ca2+ was applied (black; n = 5), indicated by the bar. Likewise, the K60E mutation in CRACM3 completely abolished current activation (red; n = 5). Error bars indicate SEM. (E and F) Representative Co-IP analysis of HEK-293 cells transfected with FLAG-myc-CAD construct and various short N-terminal constructs of CRACM1 (E) or CRACM3 (F), respectively. Immunoblotting of the corresponding lysates is shown in the left row. Lines represent control experiments of cells transfected with the CAD construct only (∼14 kD), no transfected HEK293 cells, and the short N-terminal construct of CRACM1 or CRACM3 (∼32 kD) alone or cotransfected with the CAD construct. Blots in the upper lane were immunoblotted with anti-GFP antibody, and those in the lower lane were immunoblotted with anti-FLAG antibody. The upper right lane shows the actual Co-IP, using agarose beads coated with FLAG antibody. For both CRACM1 and CRACM3, all three N-terminal constructs bound to CAD. The lower right lane represents the immunoprecipitated fraction immunoblotted with anti-FLAG. The arrow indicates the band representing the light chain of the FLAG antibody.