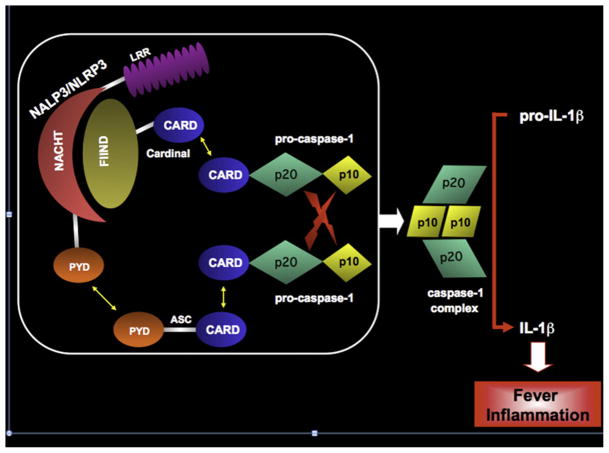
FIG 1.
The NALP3 (NLRP3) inflammasome and IL-1β–activating platform. The NALP3 (NLRP3) inflammasome is a macromolecular complex that cleaves pro–IL-1β to its biologically active form by bringing 2 molecules of pro–caspase-1 in close apposition with one another. NALP3/NLRP3, also known as cryopyrin, is comprised of an N-terminal pyrin domain (PYD), a NACHT domain, and a C-terminal leucine-rich repeat (LRR). The PYD of NALP3/NLRP3 binds the N-terminal PYD of the adaptor protein ASC (apoptosis-associated speck-like protein with a caspase recruitment domain) by homotypic interactions. The C-terminal caspase recruitment domain (CARD) of ASC binds the N-terminal CARD of pro–caspase-1, again through homotypic interactions. The NACHT domain of NALP3/NLRP3 binds the N-terminal FIIND domain (domain with function to find) of Cardinal. The C-terminal CARD of Cardinal recruits a second molecule of pro–caspase-1 to the complex. With induced proximity, the 2 pro–caspase-1 molecules undergo autocatalysis, liberating 2 catalytically active p20 and 2 catalytically active p10 domains, which form a heterotetramer capable of cleaving pro–IL-1β.