Table 2.
Au(I)-Catalyzed Arene Synthesis: Propargyl Ester Scope
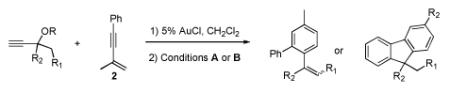 | ||||||
|---|---|---|---|---|---|---|
| entry | propargyl ester | cp yielda | conditions Ab | yieldc | conditions Bb | yieldc |
| 1 |
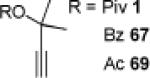
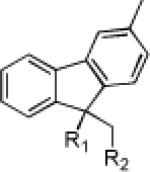
|
5 80% | 3 | 89% | 4 | 76% |
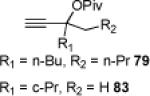
| 2 | 68 82% | 79% | 19% (+ 53% 3) | |||
| 3 | 70 60% | 81% | 57% (+ 32% 3) | |||
|
| ||||||
| 4 |
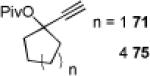
|
72 80% |
|
73 89% |
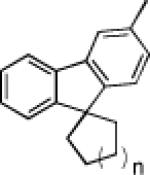
|
74 63% |
| 5 | 76 83% | 77 91% | 78 66% | |||
|
| ||||||
|
|
|
||||
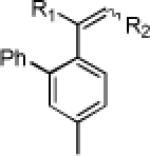
| 6 | 80 59% | 81 77% (8.3:1)d | 82 84% | |||
| 7 | 84 79% | 85 72% | 86 84% | |||
Isolated yields of cis-cyclopropane. Reactions run with 3:1 ratio of propargyl ester:2
A: 5% AgOTf, 5% (ArO)3PAuCl, CH2Cl2. B: 5% AgSbF6, 5% (ArO)3PAuCl, CH2Cl2.
Isolated yields
E:Z ratio.
