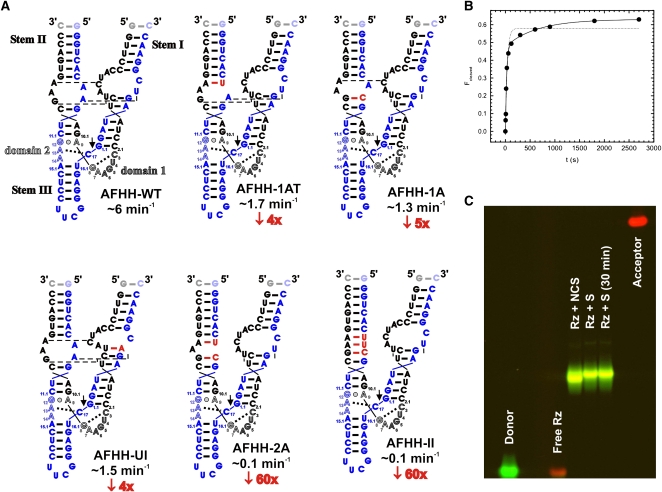
FIGURE 1.
Design and properties of AFHH hammerhead ribozyme and its variants. (A) Cleavage rate constants of AFHH variants with mutations to the loop regions. “Wild-type” AFHH-WT is shown in the top left. For each mutant ribozyme, the nucleotides that have been modified are highlighted in red. Observed cleavage rate constants are shown (blue) along with the relative reduction in rate (red) compared to AFHH-WT. All cleavage assays were conducted under standard conditions: 50 mM Tris-HCl (pH 8.0), 25°C, 1 mM MgCl2. (B) Representative cleavage assay time course of the chemically synthesized AFHH-WT ribozyme under standard conditions: 50 mM Tris-HCl (pH 8.0), 25°C, 1 mM MgCl2. Single (dashed line) and double (solid line) exponential fits are shown, with the double exponential giving a better fit (R2 of 0.99 vs. 0.97). The observed rate constant for the fast phase is 2.4 min−1 (fraction cleaved: 50%), that of the slow phase is 0.09 min−1 (15%). (C) Testing for structural homogeneity of the AFHH-WT ribozyme–substrate complex using a FRET-based electrophoretic mobility shift assay. The ribozyme strand (Rz) and noncleavable (NCS) or cleavable (S) substrate strands were annealed and run on a 10% nondenaturing polyacrylamide gel as described in Materials and Methods. The complex loaded into the “Rz+S (30 min)” lane was incubated for an additional 30 min at 37°C after the standard annealing protocol. Donor emission is shown in green, acceptor emission in red.