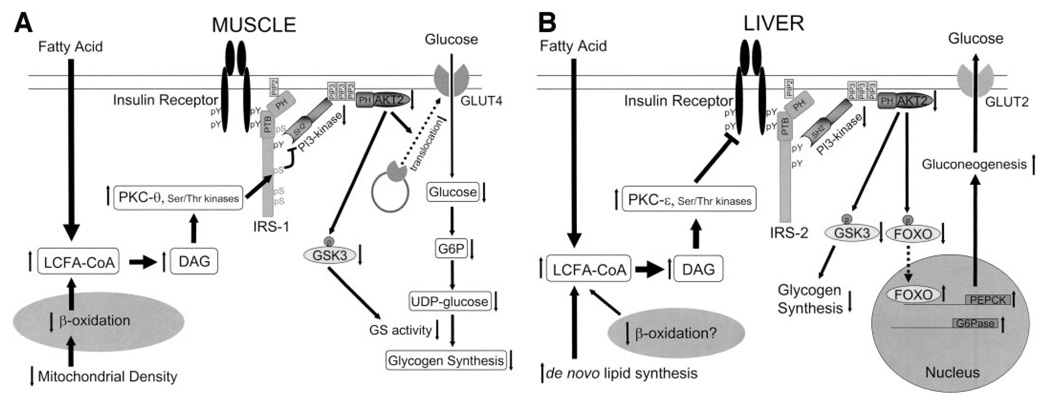
FIG. 1.
The molecular mechanism of fat-induced insulin resistance in skeletal muscle (A) and liver (B). A: Increases in intramyocellular fatty acyl CoAs and diacylglycerol due to increased delivery from plasma and/or reduced β-oxidation due to mitochondrial dysfunction activate serine/threonine kinases such as protein kinase C (PKC-θ rodents, PKC-β and -δ humans) in skeletal muscle. The activated kinases phosphorylate serine residues on IRS-1 and inhibit insulin-induced PI 3-kinase activity, resulting in reduced insulin-stimulated AKT2 activity. Lowered AKT2 activity fails to activate GLUT4 translocation, and other downstream AKT2-dependent events, and consequently insulin-induced glucose uptake is reduced. B: Increases in hepatic diacylglycerol content due to increased delivery of fatty acids from the plasma and/or increased de novo lipid synthesis and/or reduced β-oxidation activate protein kinase C-ε (and potentially other serine kinases), leading to reduced insulin receptor kinase activity and reduced IRS-2 tyrosine phosphorylation, resulting in reduced insulin stimulation of glycogen synthase activation and decreased phosphorylation of forkhead box protein O (FOXO), leading to increased hepatic gluconeogenesis. DAG, diacylglycerol; PTB, phosphotyrosine binding domain; PH, pleckstrin homology domain; SH2, src homology domain; GSK3, glycogen synthase kinase-3.