Abstract
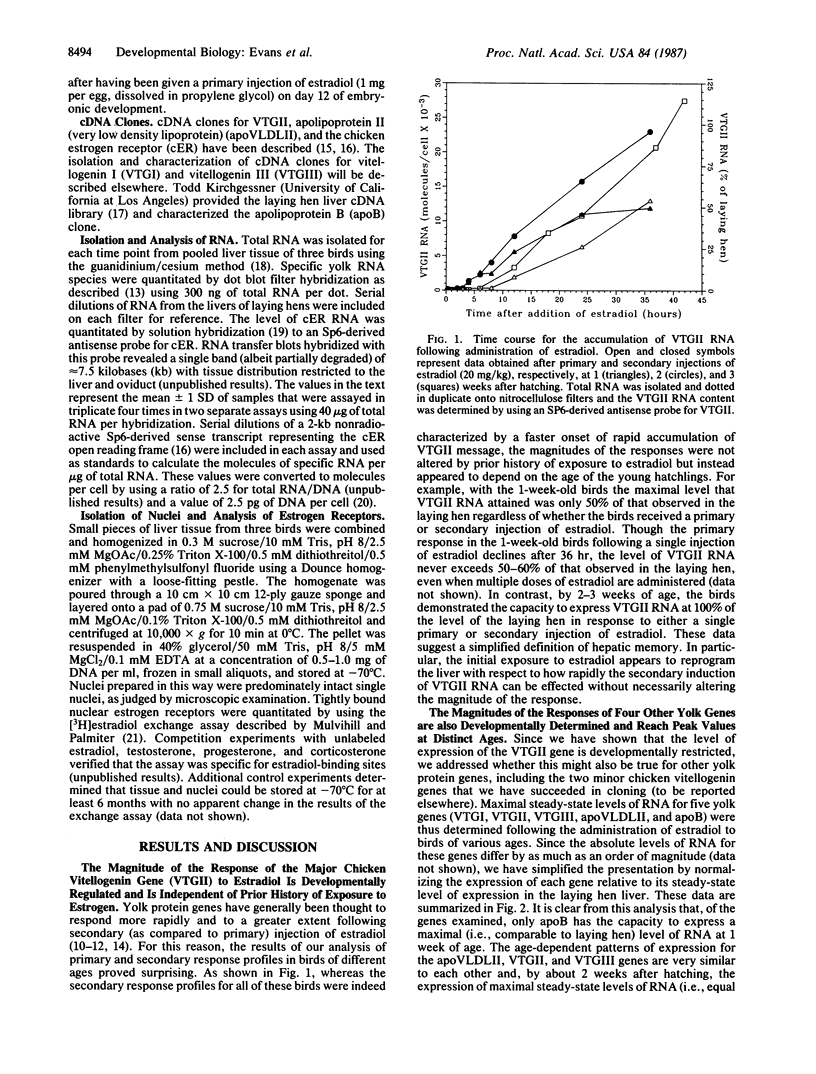
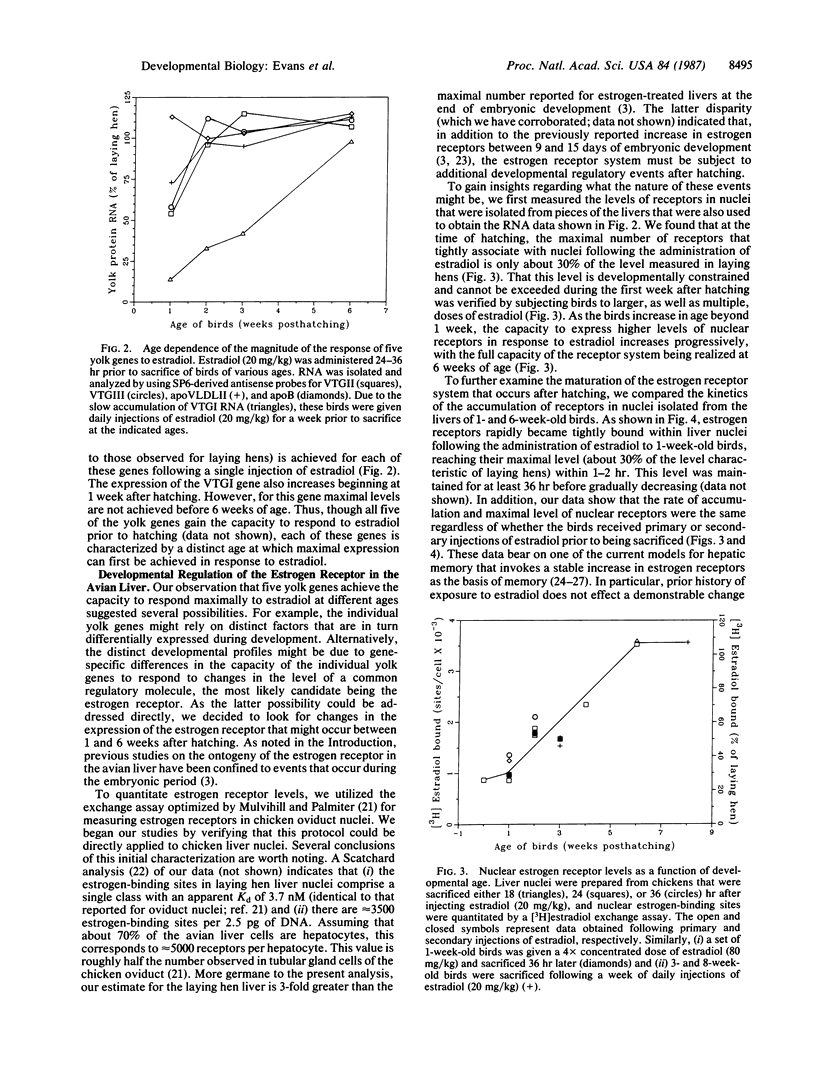
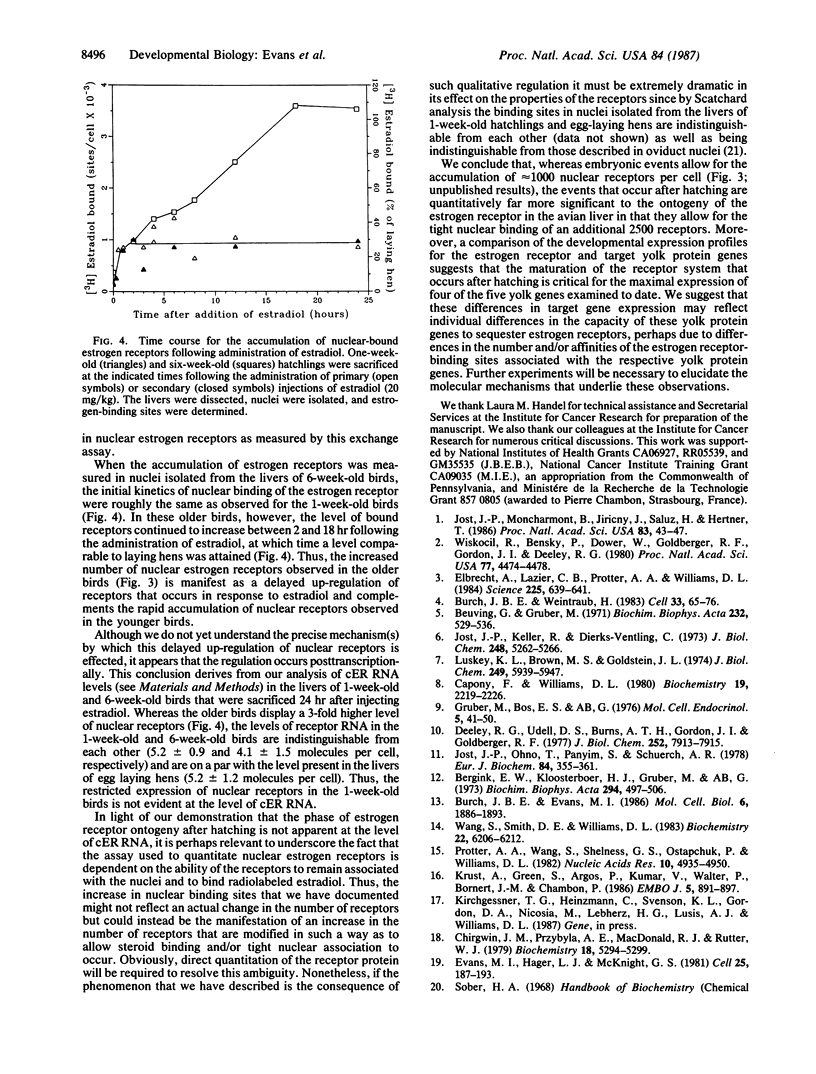
The magnitude of the expression of five yolk protein genes in the avian liver in response to exogenous estradiol is shown to be developmentally regulated. Though each of these yolk protein genes gains the capacity to respond to estradiol during embryonic development, we demonstrate that maximal responses for the different genes are achieved at distinct ages between 1 and 6 weeks after hatching. This observation prompted us to look for possible correlations between yolk protein gene expression and changes in the expression of estrogen receptors that might also occur after hatching. We discovered that indeed the maximal level of nuclear estrogen receptors (assayed following the administration of estradiol) increases progressively over this same period of development from approximately 1000 receptors per cell at 1 week after hatching to approximately 3500 receptors per cell at 6 weeks after hatching. The latter number represents the fully mature state, as comparable levels of receptors are present in the livers of egg-laying hens. Thus, though increases in the expression of estrogen receptors during embryonic liver development have previously been reported, our results indicate that the changes that occur after hatching are quantitatively far more significant to the developmental program for this transcription factor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergink E. W., Kloosterboer H. J., Gruber M., Ab G. Estrogen-induced phosphoprotein synthesis in roosters. Kinetics of induction. Biochim Biophys Acta. 1973 Feb 4;294(1):497–506. doi: 10.1016/0005-2787(73)90105-6. [DOI] [PubMed] [Google Scholar]
- Beuving G., Gruber M. Induction of phosvitin synthesis in roosters by estradiol injection. Biochim Biophys Acta. 1971 Mar 25;232(3):529–536. doi: 10.1016/0005-2787(71)90607-1. [DOI] [PubMed] [Google Scholar]
- Burch J. B., Evans M. I. Chromatin structural transitions and the phenomenon of vitellogenin gene memory in chickens. Mol Cell Biol. 1986 Jun;6(6):1886–1893. doi: 10.1128/mcb.6.6.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Capony F., Williams D. L. Apolipoprotein B of avian very low density lipoprotein: characteristics of its regulation in nonstimulated and estrogen-stimulated rooster. Biochemistry. 1980 May 13;19(10):2219–2226. doi: 10.1021/bi00551a035. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Udell D. S., Burns A. T., Gordon J. I., Goldberger R. F. Kinetics of avian vitellogenin messenger RNA induction. Comparison between primary and secondary response to estrogen. J Biol Chem. 1977 Nov 25;252(22):7913–7915. [PubMed] [Google Scholar]
- Elbrecht A., Lazier C. B., Protter A. A., Williams D. L. Independent developmental programs for two estrogen-regulated genes. Science. 1984 Aug 10;225(4662):639–641. doi: 10.1126/science.6740331. [DOI] [PubMed] [Google Scholar]
- Evans M. I., Hager L. J., McKnight G. S. A somatomedin-like peptide hormone is required during the estrogen-mediated induction of ovalbumin gene transcription. Cell. 1981 Jul;25(1):187–193. doi: 10.1016/0092-8674(81)90243-9. [DOI] [PubMed] [Google Scholar]
- Gruber M., Bos E. S., Ab G. Hormonal control of vitellogenin synthesis in avian liver. Mol Cell Endocrinol. 1976 Jun-Jul;5(1-2):41–50. doi: 10.1016/0303-7207(76)90069-1. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W. A cytoplasmic high affinity estrogen-binding protein in the embryonic chicken liver. Eur J Biochem. 1977 Nov 1;80(2):461–468. doi: 10.1111/j.1432-1033.1977.tb11901.x. [DOI] [PubMed] [Google Scholar]
- Hayward M. A., Mitchell T. A., Shapiro D. J. Induction of estrogen receptor and reversal of the nuclear/cytoplasmic receptor ratio during vitellogenin synthesis and withdrawal in Xenopus laevis. J Biol Chem. 1980 Dec 10;255(23):11308–11312. [PubMed] [Google Scholar]
- Joss U., Bassand C., Dierks-Ventling C. Rapid appearance of estrogen receptor in chick liver nuclei: partial inhibition by cycloheximide. FEBS Lett. 1976 Jul 15;66(2):293–298. doi: 10.1016/0014-5793(76)80525-x. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Keller R., Dierks-Ventling C. Deoxyribonucleic acid and ribonucleic acid synthesis during phosvitin induction by 17beta-estradiol in immature chicks. J Biol Chem. 1973 Aug 10;248(15):5262–5266. [PubMed] [Google Scholar]
- Jost J. P., Moncharmont B., Jiricny J., Saluz H., Hertner T. In vitro secondary activation (memory effect) of avian vitellogenin II gene in isolated liver nuclei. Proc Natl Acad Sci U S A. 1986 Jan;83(1):43–47. doi: 10.1073/pnas.83.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost J. P., Ohno T., Panyim S., Schuerch A. R. Appearance of vitellogenin mRNA sequences and rate of vitellogenin synthesis in chicken liver following primary and secondary stimulation by 17 beta-estradiol. Eur J Biochem. 1978 Mar 15;84(2):355–361. doi: 10.1111/j.1432-1033.1978.tb12175.x. [DOI] [PubMed] [Google Scholar]
- Krust A., Green S., Argos P., Kumar V., Walter P., Bornert J. M., Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986 May;5(5):891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskey K. L., Brown M. S., Goldstein J. L. Stimulation of the synthesis of very low density lipoproteins in rooster liver by estradiol. J Biol Chem. 1974 Sep 25;249(18):5939–5947. [PubMed] [Google Scholar]
- Mulvihill E. R., Palmiter R. D. Relationship of nuclear estrogen receptor levels to induction of ovalbumin and conalbumin mRNA in chick oviduct. J Biol Chem. 1977 Mar 25;252(6):2060–2068. [PubMed] [Google Scholar]
- Protter A. A., Wang S. Y., Shelness G. S., Ostapchuk P., Williams D. L. Isolation and characterization of a cDNA clone specific for avian vitellogenin II. Nucleic Acids Res. 1982 Aug 25;10(16):4935–4950. doi: 10.1093/nar/10.16.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westley B., Knowland J. Estrogen causes a rapid, large and prolonged rise in the level of nuclear estrogen receptor in Xenopus laevis liver. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1167–1172. doi: 10.1016/0006-291x(79)91531-6. [DOI] [PubMed] [Google Scholar]
- Wiskocil R., Bensky P., Dower W., Goldberger R. F., Gordon J. I., Deeley R. G. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]



