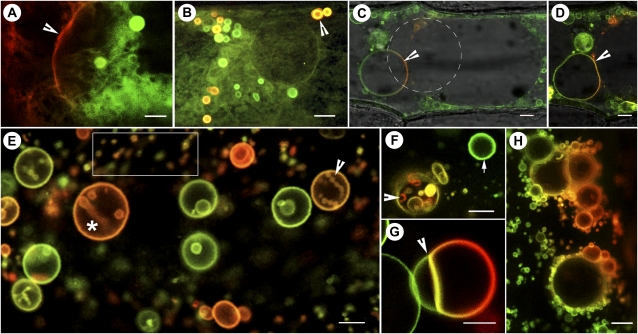
Figure 2.
mEosFP-based probes targeted to different membrane compartments. A, A region lying between two onion epidermal cells transiently expressing the mEosFP::PIP1 probe shows the nonconverted (green) and the photoconverted (red) labeling of tubular-vesicular membranes. The arrowhead points to the plasma membrane. (See also Supplemental Fig. S1.) B, Membrane tubules and multilamellar vesicles (arrowhead) in a mEosFP::PIP1-expressing cell. Vesicles are motile, and the red (photoconverted) mix readily with nonconverted (green) vesicles within minutes. The merged image acquired from both green and red channels is a single scan along the xy axis. Due to the rapid motility of vesicles and tubules, separate preconversion and postconversion images from green and red channels do not exhibit the vesicles observed here and can only inform about the nonvisibility of the probe in the red channel prior to its photoconversion. C and D, An onion epidermal cell transiently expressing the mEosFP::α-TIP1 probe shows vacuolar membranes being highlighted. The photoconverted portion of membrane (arrowheads) delimiting a minivacuole appears red and shows a regular spherical shape (C) that changes into an ellipsoid form in a subsequent scan (D). The merged images are sufficient to show dynamic changes in the highlighted compartment like conventional FPs, but through sequential time-lapse imaging they have the potential to inform about membrane dynamics within the vacuole compartment. E, Differential labeling of various PI(3)P-enriched vesicles following transient expression of mEosFP::2xFYVE in an onion epidermal cell. Based on their size, the smaller vesicles (e.g. boxed area) qualify as endosomes, while the larger vesicles (asterisk) are considered prevacuoles and vacuoles. The number of endosomes is fairly representative of onion cells exhibiting active cytoplasmic streaming. A number of prevacuoles (e.g. arrowhead and asterisk) display internalized vesicles. Full photoconversion labels vesicles red, whereas nonconverted vesicles remain green. Partial conversion results in labeling hues ranging from green to red. F, A single mEosFP::2xFYVE-labeled vesicle (arrowhead) illuminated with a 3-s pulse of violet-blue light exhibits internalized vesicles of different colors. This suggests that sequential photoconversion pulses may be used to determine the relative differences in protein content or membrane labeling between vesicles. The arrow points to an unconverted vesicle used as a control. G, Rapid membrane fusion observed along a line of contact between photoconverted and nonphotoconverted vesicles (arrowhead) achieved through transient salt-induced plasmolysis suggests the usefulness of the mEosFP::2xFYVE probe for understanding homotypic vesicle fusion. H, A PEG-treated cell exhibiting aggregation of numerous small mEos::2xFYVE vesicles around the larger ones. This state is maintained until PEG is removed, when rapid vesicle fusion occurs. All images were acquired using microscopy system 2. Bars = 5 μm (A–D and F–H) and 1 μm (E).