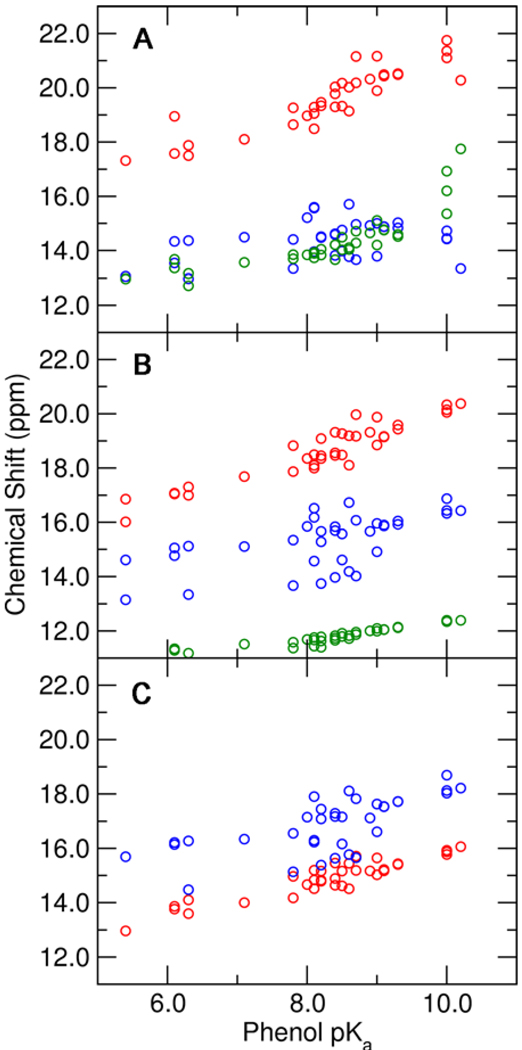
Figure 9.
Calculated NMR chemical shifts for the protons in the Tyr16-phenolate hydrogen bond (red), the Asp103-phenolate hydrogen bond (blue), and the Tyr16-Tyr57 hydrogen bond (green) as functions of the solution pKa of the substituted phenolate. These chemical shifts were determined from QM/MM calculations of the phenolate bound to pKSI D40N with (A) the unmodified hydrogen-bonding network, (B) the Y32F mutation, and (C) the Y32F/Y57F mutations. All calculations included Tyr16, Asp103, Asp40Asn, residue 32, residue 57, and the phenolate in the QM region.