Abstract
Memory T cells are generated following an initial viral infection, and have the potential for mediating robust protective immunity to viral re-challenge due to their rapid and enhanced functional responses. In recent years, it has become clear that the memory T cell response to most viruses is remarkably diverse in phenotype, function, and tissue distribution, and can undergo dynamic changes during its long-term maintenance in vivo. However, the role of this variegation and compartmentalization of memory T cells in protective immunity to viruses remains unclear. In this review, we discuss the diverse features of memory T cells that can delineate different subsets, the characteristics of memory T cells thus far identified to promote protective immune responses, and how the heterogeneous nature of memory T cells may also promote immunopathology during antiviral responses. We propose that given the profound heterogeneity of memory T cells, regulation of memory T cells during secondary responses could focus the response to participation of specific subsets, and/or inhibit memory T-cell subsets and functions that can lead to immunopathology.
Introduction
The promise that manipulation of memory T cells holds for providing long-lasting protective immunity against viral infections is matched by the challenge of understanding their complex and heterogeneous properties. In primary responses to newly encountered viruses, naive T cells become activated in lymphoid tissue and differentiate into effector T cells, which then migrate to peripheral sites and coordinate viral clearance. Most of these effector cells die after virus is cleared, although a subset of primed, virus-specific T cells develops into long-lived memory. These memory T cells can mediate rapid and effective recall immune responses conferring protective immunity to viral challenge. There are two main features of antigen-specific memory T cells that distinguish them from naive T cells and enable them to coordinate efficient secondary responses. One feature is the ability of memory T cells to mediate rapid effector responses upon antigenic recall, compared to naive T cells that lack immediate effector function. The other feature is the remarkable heterogeneity of memory T cells in homing capacities, function, and tissue distribution in lymphoid and non-lymphoid sites—starkly contrasting the homogenous phenotype and exclusive lymphoid residence of naive T cells. This memory heterogeneity imparts a functional and spatial diversity to the recall response; however, the role of heterogeneous memory T cells in secondary responses and protective immunity to viral challenge remains poorly understood. In particular, it is not known whether maximizing memory heterogeneity or focusing a memory T-cell response is more beneficial to protective immunity. In this review, we will discuss heterogeneous properties of memory T cells, how specific memory T-cell subsets differ in their antiviral efficacy, and how functional diversity may lead to both protection and immunopathology in antiviral responses. We also propose that optimizing protective immunity by virus-specific memory T cells can be achieved by regulating the heterogeneity of existing memory T-cell populations.
Memory T cells: Basic properties
Memory T cells exhibit enhanced functional properties and distinct phenotypic features, compared to naive T cells (60,132). Functionally, memory T cells exhibit rapid effector cytokine production within hours of stimulation, whereas naive T cells require days of sustained activation to differentiate into effector cytokine producers (26,60). This “rapid recall” response is the defining feature of memory CD4 and CD8 T cells, and includes production of Th-1-type effector cytokines IFN-γ, IL-2, and TNF-α; Th-2-like cytokines IL-4, IL-5, and IL-10 (93); or the Th-17-type cytokine IL-17 (148,149). Memory T cells also have less stringent activation requirements compared to naive T cells, including a reduced activation threshold for low antigen doses (114). Memory T cells can be fully activated by many antigen-presenting cell (APC) types such as resting B cells, macrophages, and endothelial cells (31,37), as well as dendritic cells (DCs) (166), which are the primary APCs for activating naive T cells. Phenotypically, memory T cells differ from naive T cells in their elevated expression of adhesion markers CD44 (22) and CD11a (147) or CD45RO in humans. The CD45RB isoform was originally found to be differentially expressed on naive versus memory CD4 T cells (19,73); however, CD45RB expression occurs on a subset of memory CD4 T cells (16) (Table 1), and the human counterpart, CD45RA, can also be expressed on subpopulations of memory T cells (131). Thus, the two invariant functional and phenotypic features that define memory T cells are rapid effector function, and increased CD44 expression in mice or CD45RO expression in humans.
Table 1.
Phenotypic Variations that Define Functional Subsets of Memory T Cells
| Phenotypic markers | Memory subsets | Functions | References |
|---|---|---|---|
| CCR7 | Effector-memory | Effector cytokine production | 6,118–120,141 |
| CD62L | CD62Llo/CCR7− Central-memory CD62Lhi/CCR7+ |
IL-2, high proliferation | |
| CD45RB | CD45RBhi | Low effector responses | 16 |
| CD45RBlo | Effector cytokines | ||
| VLA1 | CD49b+ | TNF-α, protective | 48, 62 |
| VLA-2 (CD49b) | CD49b− | IL-10 | |
| VLA-1+ | Th-1 functions | ||
| CD27 | Effector memory | Effector cytokine production | 18, 46, 53 |
| CD27lo Central memory CD27hi |
IL-2, high proliferation | ||
| CD28 | CD28+ | All resting memory | 98, 106, 150 |
| CD28− | Newly activated memory/CMV downmodulation | ||
| CD43 | CD43lo | Effector cytokines | 53, 71, 100 |
| CD43hi | Naive | ||
| CCR6, CCR4 | CCR6+CCR4+ | IL-17 | 2, 9 |
| CXCR3 | CXCR3+ | Th-1 responses | 68, 131 |
Memory T-Cell Heterogeneity
The profound heterogeneity of memory T cells first became apparent 8 years ago in studies by several groups (5,6,119,120,141). This heterogeneity was found in both mouse and human CD4 and CD8 memory T cells, and was defined by variations in expression of the lymph node homing receptors CD62L and/or CCR7 (6,120,141), and diverse distribution in lymphoid and non-lymphoid tissue sites (86). Lanzavecchia and colleagues defined two subsets of memory T cells in human peripheral blood based on CD62L/CCR7 expression and functional capacity, with the CCR7+CD62Lhi population producing predominantly IL-2 designated as “central -memory” (TCM), and the CCR7–CD62Llo subset producing effector cytokines defined as “effector-memory” (TEM) (118–120). Central and effector memory subsets also were found to delineate memory subsets with distinct tissue distribution, with TCM residing primarily in lymphoid sites and peripheral blood, and TEM predominating in non-lymphoid sites and mucosal compartments (75,86,118). Further in vitro analysis of human TCM and TEM subsets led to a model in which the TCM subset served as a continuously renewing “memory stem cell” which also replenished the TEM pool (72).
Although the TEM/TCM concept and nomenclature has been widely adopted, there is accumulating in vivo evidence that the function, heterogeneity, and lineage relationship of memory T-cell subsets do not follow the central/effector memory paradigm. First, the original functional dichotomy between TCM and TEM subsets does not apply to multiple antigen-specific models. Equivalent effector function was found to be produced by mouse LCMV-specific TCM and TEM CD8 T cells (139,155), CD62Llo subsets of mouse memory CD4 T cells (16), and similar effector cytokine functions in human virus and antigen-specific TEM and TCM subsets (25,36,118,134,135), indicating that antigen-specific memory subsets may not have intrinsic differences in cytokine production. Second, phenotypic heterogeneity of memory T cells is not limited to CD62L/CCR7 expression, and the expression of activation markers, adhesion molecules, homing receptors, co-stimulatory receptors, and chemokine receptors has been shown to delineate memory subsets with distinct functions (Table 1).For example, differences in the expression of integrins CD49b (VLA-2) (62) and VLA-1 (48) define functional subsets of memory CD4 T cells in mice and humans, respectively, with VLA+ memory T cells exhibiting more Th-1-like functions (Table 1).The co-stimulatory molecules CD28 and CD27 are differentially expressed by human and/or mouse memory subsets (46,115). While CD28 is expressed by most resting effector and central memory T cells (17,44,98), a proportion of human CD28− memory T cells has been detected in the periphery and is associated with aging or chronic infection (109,150). Variations in CD27 expression also occur on human and mouse memory T cells, with the CD27lo phenotype on memory CD8 T cells in mucosal sites denoting lytic capacity (18,53,84). In addition, coordinate expression of CD27 and the adhesion molecule CD43 together define functional subsets of mouse memory CD8 T cells with different recall and proliferative capacities (53). Human memory T cell subsets can also be distinguished by variations in CD43 expression that likewise correlate to different functional capacities (71,100), independent of CD62L expression (Table 1).Therefore, phenotypic classification into TEM and TCM subsets, which is based on CD62L or CCR7 homing receptor expression, does not fully describe the phenotypic and functional complexities of a given memory T-cell population.
The expression of chemokine receptors, which control leukocyte migration to tissue sites, inflammation, and interactions with immune accessory cells (70), also exhibit considerable variation on memory T-cell populations and can define functional subsets (Table 1).The original TCM and TEM subsets were defined based on expression of the CCR7 chemokine receptor (119,120), that mediates lymphoid homing similar to CD62L. However, the coordinate expression of CCR7 and CD62L on TCM and their downregulation on TEM does not occur on virus-specific memory CD8 T cells in mice (110,140), and in subsequent studies, CCR7 did not distinguish functional subsets of virus-specific memory T cells in humans and mice (25,35,139). The expression of other types of chemokine receptors can delineate subsets of memory T cells with distinct cytokine profiles and replicative history, as defined by telomere length. Thus, expression of CXCR3 and/or CCR4 can define subsets of memory CD4 T cells having the capacity to produce Th-1 or Th-2 cytokines, and also delineate subsets with different in vivo turnover (68,111,131). CCR8 expression on memory T cells can indicate the potential for production of Th-2 cytokines (130), and recently, expression of both CCR6 and CCR4 has been shown to mark a population of memory CD4 T cells that produce the proinflammatory cytokine IL-17 (2,9). It is not known whether expression of chemokine receptors directly controls functional capacity, or rather reflects stimulation, trafficking, or replicative history of memory T cells that has more directly biased their cytokine profile. These broad variations in surface marker expression on memory T cells suggest a diverse usage of trafficking markers that may alter during subsequent recall to antigen challenge.
The compartmentalization of memory T cells in diverse tissue sites adds yet another layer to the phenotypic and functional complexity of memory T cells described above. While the majority of memory T cells in non-lymphoid tissue bear a predominant CD62Llo profile (16,85–87), there is increasing evidence that tissue-resident memory T cells exhibit compartment-specific phenotypic, functional, and homing properties. For example, mouse bone marrow (BM)-resident memory CD8 T cells exhibit enhanced effector and proliferative capacities (14,105) as we also found for human BM memory T cells (169). Lung memory T cells, by contrast, exhibit reduced proliferation yet highly activated phenotypes and effector responses (24,41,42,113,159). Gut-resident memory T cells exhibit further variations in phenotypes, homing, and increased apoptosis (76,88). In addition, we have found that lung and spleen memory CD4 T cells exhibit tissue-specific homing tropism (16), indicating that certain tissue compartments can impart specific properties on their indigenous memory T cells. In addition, CCR7+/CD62L+ TCM-phenotype cells can be found in non-lymphoid sites including lung (16,101), CNS (65), and gut (145,146), and these non-lymphoid CD62Lhi cells are not functionally equivalent to lymphoid CD62Lhi subsets (16,146). When taken together, these results indicate that the memory subsets in spleen and peripheral blood are not equivalent to memory T cells of comparable CD62L phenotype resident in peripheral tissue parenchyma. A given memory T-cell population therefore consists of multiple functional subtypes in diverse tissue sites that are maintained by homeostasis, and may recirculate between tissue sites or turnover within each compartment (Fig. 1A). The functional properties of individual memory T cells are influenced by a combination of surface marker expression and tissue compartment, although the precise contributions of these factors in directing memory T-cell responses in vivo are unknown.
FIG. 1.
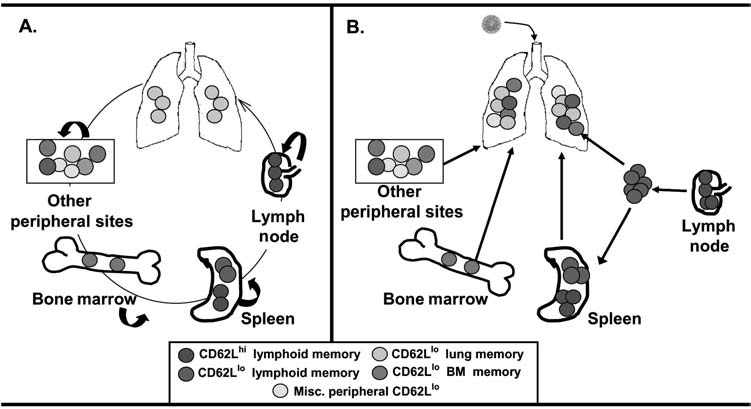
Schematic model of heterogeneous memory T cells during steady-state conditions and in response to a respiratory infection. (A) During steady-state conditions, memory T cells in different tissue sites are maintained by homeostasis, with some replenishment from lymphoid sites, and possible trafficking between distinct non-lymphoid sites. (B) During a site-specific infection, as in the lung, memory T cells from multiple lymphoid and non-lymphoid sites can potentially become activated and migrate to the infectious site, resulting in a rapid and heterogeneous response. The participation of multiple subsets and their potential for mediating protective immunity and/or immunopathology are discussed in the text.
The lineage relationship between CD62L memory T-cell subsets and lymphoid and non-lymphoid memory T cells also remains an unresolved issue. In particular, it is not known whether memory subset delineation is established during priming, and/or varies over time during memory maintenance (95). The CD62L profile of memory T cells can be affected by initial priming conditions, including the extent of antigenic stimulation during priming and the antigen-specific precursor frequency (74,95). For CD4 T cells, strong or sustained antigenic stimulation during priming yielded memory T cells with increased effector function (80) and increased CD62L heterogeneity (94), whereas for CD8 T cells, limiting the extent of antigen exposure during infection resulted in more CD62Lhi lymphoid memory CD8 T cells (143,156). The precursor frequency of antigen-specific CD8 T cells also affects the development of heterogeneous memory cells. A low precursor frequency responding to vesicular stomatitis virus (VSV) infection gave rise to primarily CD62Llo cells, whereas higher T-cell precursors generated more CD62Lhi TCM cells (13,83). The role of CD4 T-cell precursors in heterogeneous memory generation during viral infection is not yet defined, although quantitation of peptide-specific CD4 T-cell precursors based on new MHC class II tetramer reagents revealed correlations between initial naive precursor and memory cell frequency (92). CD62L expression can also vary over time during memory persistence in the periphery (112,155), or upon homing to lymphoid or non-lymphoid tissue sites (16,84). At present, it remains unknown whether the tissue-specific influences on resident memory T cells are reversible or inducible upon exit or entry into a specific compartment, respectively. The factors that regulate CD62L expression and homing capacity of memory T cells in vivo remain unresolved issues and are important parameters for understanding the lineage relationship of CD62L memory subsets.
In summary, although the use of TEM or TCM predominates in the literature, this classification does not account for the multiple variations in phenotype, function, and homing of peripheral memory T cells, as well as tissue-specific variations that exist in non-lymphoid memory populations. In addition, designating memory subsets as TCM or TEM implies a lineage relationship that is not yet established. Thus, we propose that a more accurate designation of memory subsets would be to indicate both the tissue of residence and homing receptor phenotype, such as LN-CD62Lhi, lung-CD62Llo, or spleen-CD62Llo memory T cells. This designation uses the CD62L phenotype to indicate lymphoid homing capacity, and the tissue or origin to indicate potential tissue-specific influences, and makes no assumptions regarding lineage relationships.
Memory T-Cell Heterogeneity and Protective Immunity
The heterogeneous nature of memory T cells raises important questions concerning the role of this memory diversity in antiviral protective immunity. In recall responses to viral infection, multiple types of memory subtypes can participate—including memory T cells at the site of infection, lymphoid memory T cells that become activated and migrate to peripheral sites, and memory T cells circulating from other non-lymphoid compartments, with each of these memory subtypes exhibiting specific functional and proliferative capacities (Fig. 1B). The different types of memory T cells required for recall responses to specific viruses are important issues that need to be resolved in order to promote long-term T-cell immunity. While heterogeneous memory T cells may diversify the recall response, leading to more effective protective immunity, it is possible that only specific subpopulations of memory T cells within the heterogeneous pool can mediate protective responses. However, the existence of heterogeneous memory T-cell subsets may also result in certain subsets of memory T cells being ineffective or potentially detrimental to antiviral protective immunity.
Adoptive transfer approaches have been informative in assessing the in vivo protective capacities of specific memory subsets, and have revealed that certain memory subsets may be more effective than others in mediating viral clearance. The protective capacities of central and effector memory subsets differing in CD62L expression vary depending on the viral infection system. In LCMV infection, purified splenic CD8 TCM cells transferred into adoptive mouse hosts mediated more effective viral clearance than purified TEM (155), whereas for Sendai virus infection, the protective capacity of memory CD8 T-cell subsets varied over time and did not depend on CD62L profile (53,112). By contrast, protection from vaccinia virus infection was restricted to the TEM subset of CD8 T cells (12). For CD4 T-cell subsets, less is known concerning their protective capacity in viral systems. CD4 T cells have been shown to provide protection against a number of different viruses, including respiratory viruses (57), rotavirus (89), gamma-herpes viruses (29), and picornaviruses (99). The subset specificity of protective memory CD4 T cells for most of these viruses remains undefined, although CD62Llo CD4 T cells have been shown to be protective in responses to rotavirus (128), and vaccinia virus (1). Further refinements in the phenotypic and functional characterization of memory T cells should lead to insights into the type of memory T cells necessary for optimal protective immunity to viral infections.
The efficacy of a secondary T-cell response to viral infections may ultimately reside within memory T cells in specific anatomical compartments, given that viruses enter and disseminate in different tissue sites. Recent studies provide evidence that the tissue-resident memory T cells rather than the peripheral homing subtype, drive the recall response and mediate protection (63,66,157). However, the requirements for lymphoid and/or non-lymphoid memory T cells can depend on the viral system, and lymphoid memory T cells can serve important protective roles, particularly for systemic viral infection. In response to ectromelia virus (mousepox) infection, memory CD8 T cells in lymph nodes were found to be important “gatekeepers” in pathogen clearance (161). Lymphoid-derived central memory CD4 T cells have also been associated with protective responses to HIV/SIV and EBV infection. In primates vaccinated with simian immunodeficiency virus (SIV), animals that preserve their central memory pools and have strong IL-2 recall responses have lower levels of initial viral replication and prolonged survival (136). Moreover central memory CD4 T cells in blood comprise half of the responding EBV-specific memory CD4 T cells and directly replenish the effector-memory pool (52). Lymphoid memory can also predominate in response to the respiratory virus SARS, where the majority of the blood virus-specific memory CD4 T cells bear a CD62Lhi phenotype (164). However, for influenza virus infection that is restricted to the lung, memory CD8 T cells can mediate recall responses to viral challenge in mice congenitally devoid of secondary lymphoid tissue (96), suggesting that lymphoid memory T cells are dispensable for protection to respiratory pathogens. While it is likely that secondary responses to viruses at specific tissue sites involve both tissue-resident memory T cells as well as an influx of memory T cells from lymphoid and other non-lymphoid sites (Fig. 1B), whether lymphoid memory T cells mediate significant protective responses in site-specific infections remains to be determined.
Although memory T cells are found in multiple peripheral sites, those resident in the lung and lamina propria of the gut have been the focus of a number of studies due to their abundance and importance in viral infections at these sites. Lung memory CD4 and CD8 T cells persist in humans and mice following respiratory virus infection with influenza, parainfluenza, and respiratory syncytial virus (RSV) (34,45,56,102). These lung-resident memory T cells have been shown to be replenished from the peripheral pool and maintained by continuous turnover (55,84,159). Lung memory CD4 and CD8 T cells produce higher levels of IFN-γ compared to counterparts in peripheral blood or secondary lymphoid organs (16,86), and may be particularly adapted to mediate effective protective responses in situ. Hogan and colleagues demonstrated in situ rapid viral clearance when virus-specific memory CD4 T cells were administered directly to the lungs of recipient mice (57), indicating that localization of memory T cells at the site of infection is sufficient to provide protection. In the natural infection, however, influenza-specific CD8 T cells are widely dispersed, present in spleen, lung, bone marrow, and other tissue sites (82), and memory CD4 T cells specific for influenza HA are also heterogeneous in CD62L expression and tissue distribution (6,16,137). These findings indicate that multiple memory T cell subtypes could respond and home to the lung during secondary influenza challenge (Fig. 1B). Several groups have found that influenza antigens persist long after virus is cleared in the lung (59,167), and may maintain effector memory populations. Understanding the exact interplay of multiple memory subsets as well as the pathogenesis of the infecting virus would contribute significantly to targeting protective subsets during vaccination.
In the lamina propria (LP), memory T cells bear a predominant CD62Llo/CD27lo effector-memory-like phenotype, yet also exhibit distinct characteristics including up-regulated expression of the early activation marker CD69, indicating a semi-activated state, and expression of the α4β7 integrin, reflecting unique homing properties (64,67,76). These LP or mucosal memory T cells are associated with protection against intestinal viruses such as rotavirus (116). By contrast, in HIV/SIV infection, LP memory CD4 T cells are impaired in protective capacity and facilitate viral dissemination. The semi-activated state of LP memory CD4 T cells renders them highly permissive for HIV/SIV infection and severe depletion through direct infection or bystander activation with Fas-mediated apoptosis (8,20,32,76). In addition, CD4hiCD8lo double positive T cells make up a significant proportion of resident CD4 T cells in the intestines (5–20% in primates and humans), produce high levels of effector cytokines, and are highly susceptible to HIV infection (49,103,144). While dendritic cells and gut resident macrophages tend to promote an immunosuppressive state (61,129), LP memory CD4 T cells have heightened levels of CD2 and associated LIGHT expression, indicating heightened activation (30,40). Thus, the mucosal immune system, with its direct interaction with pathogens, can mediate responses highly distinct from those observed in peripheral blood or spleen. This mucosal population of memory T cells may make important contributions to antiviral immunity and may also mediate immunopathology.
Functional Heterogeneity in Memory Responses
In addition to heterogeneity in homing and tissue distribution, memory T cells also exhibit diverse capacities to produce cytokines and mediate cytolytic functions on a cellular level. Individual memory T cells can produce multiple types of cytokines rapidly following antigenic recall. Both memory CD8 and CD4 T cells have been shown to simultaneously produce IFN-γ, TNF-α, and/or IL-2 (127,154), and memory CD8 T cells also vary in expression of lytic molecules including granzyme B and perforin (138). Memory CD4 and CD8 T cells producing IL-2, IFN-γ, and/or TNF-α are referred to as “polyfunctional” memory T cells (23,33,81). Polyfunctional memory T cells can be potent antiviral T cells in that they are highly proliferative and severely limit viral replication in infected cells (50). Not all memory T cells are polyfunctional, and within a given population of virus-specific memory T cells, individual cells vary in their capacity for production of cytokines. For example, human antiviral memory CD8 T cells comprise a mixed population with most cells secreting IFN-γ alone, and a smaller number secreting both IL-2 and IFN-γ, whereas memory CD4 T-cell responses contain fairly even distributions of cells producing IL-2 or IFN-γ alone, or IL-2 and IFN-γ in combination (50). Polyfunctional responses involving IL-17 have not yet been identified, although IL-17–producing cells are exclusive of IFN-γ production in vivo (162). It has been proposed that targeting the generation of specific types of multi-functional memory T-cell clones in vaccines may be particularly advantageous for protective immunity (104). Given the complex properties of memory T cells described above, the most effective protective response will likely depend on generating the appropriate functional subtype at the appropriate tissue locale.
Functionally heterogeneous memory T cells are also endowed with plasticity in the type of recall cytokines they produce. We originally demonstrated that a population of antigen-specific memory CD4 T cells can alter the cytokine profile, depending on the nature and avidity of the recall stimulus (4,106). Similar plasticity in cytokine production has been demonstrated among human memory CD4 T cells (90), and most recently in mouse memory CD4 T cells responding to bacterial challenge in vivo (69). These findings suggest that the functional fate of memory T-cell populations is not fixed, and can be altered in different infectious environments. Inherent plasticity in memory responses to viruses has been proposed to account for adaptability in the immune responses to diverse viral antigens (124). Plasticity within memory populations can also alter productive recall responses, and therefore understanding how memory plasticity is regulated is essential to preserve the efficacious features of the anamnestic T-cell response.
Memory T Cells and Immunopathology
The rapid effector responses of memory T cells, their diverse distribution in peripheral tissue sites, and their ability to interact with tissue macrophages and endothelial cells (28,108,125) enables them to coordinate recall responses at the site of pathogen entry, but also predisposes them to be involved in immunopathology and local tissue destruction during an antiviral response. Memory CD8 T cells can mediate lethal immunopathology in response to LCMV infection (107), and destruction of lung epithelia in influenza virus infection through direct cytotoxicity and the production of TNF-α (21,160). Memory CD8 T cells have also been shown to cause severe immunopathology in responses to vaccinia virus, RSV, and LCMV (10,78,117,126). Despite their low cytotoxic potential, memory CD4 T cells can also direct responses that lead to immunopathology. Memory CD4 T cells were found to direct potent immune-mediated meningitis in LCMV-immune β2-microglobulin–deficient mice lacking CD8 T cells (54), and to promote demyelination and immunopathology during neurotropic mouse hepatitis virus infection (133). Memory CD4 T cells secreting TNF-α were also shown to promote severe tissue inflammation in LCMV, influenza, and secondary dengue virus infections (11,47,62,153). The generation of TNF-α–secreting memory T cells is therefore common in many viral diseases for which immunopathology plays a large role in tissue destruction.
The identification of memory T-cell subtypes that promote effective viral clearance with minimal immunopathology would be beneficial for optimizing memory T-cell responses during vaccinations. It will be necessary to precisely define memory T cell functions and/or subsets that lead to immunopathology. For example, memory T cells that rapidly produce high levels of IFN-γ with limited TNF-α may prove more beneficial to eliminating virus with minimal tissue damage, whereas a high level of TNF-α relative to IFN-γ production may predispose a memory T cell to mediate tissue destruction. In addition, the contribution of lymphoid versus non-lymphoid memory T cells to immunopathology may differ in responses to certain viruses. While lung resident memory T cells may promote effective protective responses to respiratory virus challenge in situ, the involvement of lymphoid memory T cells with enhanced proliferative capacities may cause increased recruitment and inflammation in the lung. By contrast, lymphoid memory T cells that are recalled in lymphoid tissue or peripheral blood may not lead to local tissue destruction. Dissecting the beneficial and detrimental functions of memory T cells and mechanisms for their actions is necessary to ensure the generation of protective T-cell vaccines.
Memory T cells generated from exposure to a pathogen can also cross-react with antigens present in an unrelated pathogen, a phenomenon termed “heterologous immunity” (122,124), which is thought to predominate in adult immune processes (27,121,151,152). Heterologous memory CD8 T cells specific for one virus can mediate immunopathology in response to an unrelated virus (27,123), suggesting that the presence of any memory T cells creates the potential for deleterious immune reactions. Thus, the enhanced and beneficial immune clearance properties of memory T cells includes the potential for increased tissue damage and immunopathology in response to viral challenge.
This dual nature of the memory T-cell response suggests that the presence of virus-specific memory T cells may not always yield a productive type of protective response, and that a given population of heterogeneous memory T cells may contain subsets with propensities for mediating immunopathology. In the case of influenza virus infection, memory T cells specific for influenza have been shown to persist in the lungs of previously infected mice and humans (34,45,56,57,158), and to be present in the peripheral blood of most older children and adults (38,43,51). However, these memory T cells are not known to provide protection in the form of sterilizing immunity to influenza. It is possible that flu-specific memory T cells participate in influenza immunity, enabling effective viral clearance; however, the immunopathology triggered by memory T cells and resultant illness masks their role. It is interesting to note that a population profoundly affected by immunopathology in the 1918 flu was adults in the 20- to 40-year-old range (39), and recent avian flu cases have had their most pathological impact in adults, with milder clinical signs in children under 5 year of age (165). While the unusual deaths of younger adults in the 1918 pandemic may be due to their naiveté to related flu strains at that period as was recently suggested (7), it is also possible that a deleterious immune reaction was triggered by memory T cells in this adult population. Further elucidation of the memory T-cell functions and subsets that promote immunopathology during viral infection is required to understand the nature of the antiviral memory T-cell response.
Optimization of Memory Heterogeneity in Antiviral Immunity
The functional, phenotypic, and spatial diversity of memory T cells and their potential for mediating immunopathology suggests that modulating memory T-cell heterogeneity could be an effective strategy for optimizing secondary responses. Targeting memory heterogeneity could be accomplished by strategies which regulate and focus memory T-cell responses to optimize their capacity for protective immunity. In general, memory T cells are believed to be resistant to regulation, as strategies that inhibit naive T-cell activation and effector generation are ineffective in the presence of memory T cells (97). For example, blockade of the CD40L/CD40 pathway, which effectively hinders primary T-cell activation, does not inhibit memory T-cell responses in vivo (3,142,168). In addition, regulatory T cells that suppress naive T-cell activation are ineffective in curtailing memory T cell–mediated rejection of allografts (163). However, there is increasing evidence that inhibition of certain co-stimulatory pathways can modulate specific memory T-cell functions and/or homing potentials. The CD28 co-stimulatory pathway was initially believed to be dispensable for memory T-cell activation based on in vitro studies (31,79); however, we and others have recently reported that CD28 signaling is required for optimal memory CD4 and CD8 T-cell secondary responses to antigenic peptides and influenza (17,98). Using an in vivo system to follow early and late recall of memory CD4 T cells in vivo, we found that inhibiting CD28 signaling on memory CD4 T cells preferentially limited antigen-driven expansion and IL-2 production, and reduced the tissue homing capacity and CD62L downregulation of memory T cells in vivo (98). CD28 has also been shown to direct homing capacity on human memory T cells (91), and we have also found that a similar change in homing capacity occurs on memory CD4 T cells responding to influenza virus (unpublished data), suggesting that CD28 co-stimulation can drive the migration of memory T cells to non-lymphoid sites. CD28 is also known to be downregulated on human memory T cells in response to viruses such as CMV (109), although the protective role of these CD28-deficient memory T cells is not yet defined. These newer findings on the role of CD28 co-stimulation in memory T-cell responses in vivo suggest that CD28 inhibition using approved biologicals such as CTLA4Ig (77) could regulate deleterious memory T-cell responses to viral infection. Other co-stimulatory pathways such as ICOS and OX40 likewise show promise in regulating primary and/or secondary antiviral responses (15,58), although their specific effects on memory T-cell functions, heterogeneity, and homing remain to be determined. Identifying additional pathways involved in memory T-cell responses will be particularly important for immunotherapeutic modulation and optimization of protective recall immunity.
Concluding Remarks
Cellular and functional heterogeneity is a universal feature of virus-specific memory T cells. Summarized below are the key features of memory heterogeneity reviewed here, their implications for antiviral protective immunity and immunopathology, and how regulation of memory T-cell heterogeneity during recall responses in vivo may be a promising strategy for optimization of protective responses.
Identification of phenotypic markers for memory T cells is constantly evolving. Although expression of certain phenotypes may be associated with specific functions, whether these phenotypes direct functional capacity remains unknown.
Memory T cells in lymphoid and non-lymphoid tissues exhibit compartment-specific features that are likely important to secondary recall to specific virus infections.
Specific memory subtypes may promote immunopathology during secondary responses, and the degree of immunopathology may depend on the tissue site, homing capacity, and functional profile of the participating memory T cells.
Modulating memory populations may be a way of directing more effective protective immunity without immunopathology.
Acknowledgments
D.L.F. is supported by National Institutes of Health grants AI050632 and AI077029, and D.V is supported by grant T32 ES007263.
References
- 1.Abate G. Eslick J. Newman FK. Frey SE. Belshe RB. Monath TP. Hoft DF. Flow-cytometric detection of vaccinia-induced memory effector CD4(+), CD8(+), and gamma delta TCR(+) T cells capable of antigen-specific expansion and effector functions. J Infect Dis. 2005;192:1362–1371. doi: 10.1086/444423. [DOI] [PubMed] [Google Scholar]
- 2.Acosta-Rodriguez EV. Rivino L. Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 3.Adams AB. Williams MA. Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadzadeh M. Farber DL. Functional plasticity of an antigen-specific memory CD4 T cell population. Proc Natl Acad Sci USA. 2002;99:11802–11807. doi: 10.1073/pnas.192263099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadzadeh M. Hussain SF. Farber DL. Effector CD4 T cells are biochemically distinct from the memory subset: evidence for long-term persistence of effectors in vivo. J Immunol. 1999;163:3053–3063. [PubMed] [Google Scholar]
- 6.Ahmadzadeh M. Hussain SF. Farber DL. Heterogeneity of the memory CD4 T cell response: persisting effectors and resting memory T cells. J Immunol. 2001;166:926–935. doi: 10.4049/jimmunol.166.2.926. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed R. Oldstone MB. Palese P. Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nat Immunol. 2007;8:1188–1193. doi: 10.1038/ni1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alimonti JB. Ball TB. Fowke KR. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virol. 2003;84:1649–1661. doi: 10.1099/vir.0.19110-0. [DOI] [PubMed] [Google Scholar]
- 9.Annunziato F. Cosmi L. Vantarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonis AF. Claassen EA. Hensen EJ. de Groot RJ. de Groot-Mijnes JD. Schrijver RS. van der Most RG. Kinetics of antiviral CD8 T cell responses during primary and post-vaccination secondary bovine respiratory syncytial virus infection. Vaccine. 2006;24:1551–1561. doi: 10.1016/j.vaccine.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann MF. Kundig TM. Hengartner H. Zinkernagel RM. Protection against immunopathological consequences of a viral infection by activated but not resting cytotoxic T cells: T cell memory without “memory T cells”? Proc Natl Acad Sci USA. 1997;94:640–645. doi: 10.1073/pnas.94.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachmann MF. Wolint P. Schwarz K. Jager P. Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 13.Badovinac VP. Haring JS. Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker TC. Coley SM. Wherry EJ. Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol. 2005;174:1269–1273. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- 15.Bertram EM. Tafuri A. Shahinian A, et al. Role of ICOS versus CD28 in antiviral immunity. Eur J Immunol. 2002;32:3376–3385. doi: 10.1002/1521-4141(200212)32:12<3376::AID-IMMU3376>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Bingaman AW. Patke DS. Mane VR. Ahmadzadeh M. Ndejembi M. Bartlett ST. Farber DL. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur J Immunol. 2005;35:3173–3186. doi: 10.1002/eji.200526004. [DOI] [PubMed] [Google Scholar]
- 17.Borowski AB. Boesteanu AC. Mueller YM, et al. Memory CD8+ T cells require CD28 costimulation. J Immunol. 2007;179:6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 18.Borst J. Hendriks J. Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17:275–281. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Bottomly K. Luqman M. Greenbaum L. Carding S. West J. Pasqualini T. Murphy DB. A monoclonal antibody to murine CD45R distinguishes CD4 T cell populations that produce different cytokines. Eur J Immunol. 1989;19:617–623. doi: 10.1002/eji.1830190407. [DOI] [PubMed] [Google Scholar]
- 20.Brenchley JM. Price DA. Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 21.Bruder D. Srikiatkhachorn A. Enelow RI. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol. 2006;19:147–155. doi: 10.1089/vim.2006.19.147. [DOI] [PubMed] [Google Scholar]
- 22.Budd RC. Cerottini JC. Horvath C. Bron C. Pedrazzini T. Howe RC. MacDonald HR. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 23.Casazza JP. Betts MR. Price DA, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cauley LS. Cookenham T. Miller TB. Adams PS. Vignali KM. Vignali DA. Woodland DL. Cutting edge: virus-specific CD4+ memory T cells in nonlymphoid tissues express a highly activated phenotype. J Immunol. 2002;169:6655–6658. doi: 10.4049/jimmunol.169.12.6655. [DOI] [PubMed] [Google Scholar]
- 25.Champagne P. Ogg GS. King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 26.Chandok MR. Farber DL. Signaling control of memory T cell generation and function. Semin Immunol. 2004;16:285–293. doi: 10.1016/j.smim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Chen HD. Fraire AE. Joris I. Brehm MA. Welsh RM. Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2:1067–1076. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- 28.Choi J. Enis DR. Koh KP. Shiao SL. Pober JS. T lymphocyte-endothelial cell interactions. Annu Rev Immunol. 2004;22:683–709. doi: 10.1146/annurev.immunol.22.012703.104639. [DOI] [PubMed] [Google Scholar]
- 29.Christensen JP. Cardin RD. Branum KC. Doherty PC. CD4(+) T cell-mediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc Natl Acad Sci USA. 1999;96:5135–5140. doi: 10.1073/pnas.96.9.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohavy O. Zhou J. Ware CF. Targan SR. LIGHT is constitutively expressed on T and NK cells in the human gut and can be induced by CD2-mediated signaling. J Immunol. 2005;174:646–653. doi: 10.4049/jimmunol.174.2.646. [DOI] [PubMed] [Google Scholar]
- 31.Croft M. Bradley LM. Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 32.Dandekar S. Pathogenesis of HIV in the gastrointestinal tract. Curr HIV/AIDS Rep. 2007;4:10–15. doi: 10.1007/s11904-007-0002-0. [DOI] [PubMed] [Google Scholar]
- 33.Darrah PA. Patel DT. De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 34.de Bree GJ. van Leeuwen EM. Out TA. Jansen HM. Jonkers RE. van Lier RA. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J Exp Med. 2005;202:1433–1442. doi: 10.1084/jem.20051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debes GF. Bonhagen K. Wolff T. Kretschmer U. Krautwald S. Kamradt T. Hamann A. CC chemokine receptor 7 expression by effector/memory CD4+ T cells depends on antigen specificity and tissue localization during influenza A virus infection. J Virol. 2004;78:7528–7535. doi: 10.1128/JVI.78.14.7528-7535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Debes GF. Hopken UE. Hamann A. In vivo differentiated cytokine-producing CD4(+) T cells express functional CCR7. J Immunol. 2002;168:5441–5447. doi: 10.4049/jimmunol.168.11.5441. [DOI] [PubMed] [Google Scholar]
- 37.Dengler TJ. Pober JS. Human vascular endothelial cells stimulate memory but not naive CD8+ T cells to differentiate into CTL retaining an early activation phenotype. J Immunol. 2000;164:5146–5155. doi: 10.4049/jimmunol.164.10.5146. [DOI] [PubMed] [Google Scholar]
- 38.Di Fabio S. Mbawuike IN. Kiyono H. Fujihashi K. Couch RB. McGhee JR. Quantitation of human influenza virus-specific cytotoxic T lymphocytes: correlation of cytotoxicity and increased numbers of IFN-gamma producing CD8+ T cells. Int Immunol. 1994;6:11–19. doi: 10.1093/intimm/6.1.11. [DOI] [PubMed] [Google Scholar]
- 39.Doherty PC. Turner SJ. Webby RG. Thomas PG. Influenza and the challenge for immunology. Nat Immunol. 2006;7:449–455. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 40.Ebert EC. CD2 activation of human lamina propria lymphocytes reduces CD3 responsiveness. Immunology. 2006;117:71–77. doi: 10.1111/j.1365-2567.2005.02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ely KH. Cookenham T. Roberts AD. Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- 42.Ely KH. Roberts AD. Woodland DL. Cutting edge: effector memory CD8+ T cells in the lung airways retain the potential to mediate recall responses. J Immunol. 2003;171:3338–3342. doi: 10.4049/jimmunol.171.7.3338. [DOI] [PubMed] [Google Scholar]
- 43.Ennis FA. Meager A. Beare AS, et al. Interferon induction and increased natural killer-cell activity in influenza infections in man. Lancet. 1981;2:891–893. doi: 10.1016/s0140-6736(81)91390-8. [DOI] [PubMed] [Google Scholar]
- 44.Farber DL. Differential TCR signaling and the generation of memory T cells. J Immunol. 1998;160:535–539. [PubMed] [Google Scholar]
- 45.Flynn KJ. Belz GT. Altman JD. Ahmed R. Woodland DL. Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 46.Fritsch RD. Shen X. Sims GP. Hathcock KS. Hodes RJ. Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol. 2005;175:6489–6497. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- 47.Gagnon SJ. Ennis FA. Rothman AL. Bystander target cell lysis and cytokine production by dengue virus-specific human CD4(+) cytotoxic T-lymphocyte clones. J Virol. 1999;73:3623–3629. doi: 10.1128/jvi.73.5.3623-3629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein I. Ben-Horin S. Li J. Bank I. Jiang H. Chess L. Expression of the α1β integrin, VLA-1, marks a distinct subset of human CD4+ memory T cells. J Clin Invest. 2003;112:1444–1454. doi: 10.1172/JCI19607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guadalupe M. Reay E. Sankaran S. Prindiville T. Flamm J. McNeil A. Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harari A. Dutoit V. Cellerai C. Bart PA. Du Pasquier RA. Pantaleo G. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 51.He XS. Holmes TH. Zhang C, et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80:11756–11766. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heller KN. Upshaw J. Seyoum B. Zebroski H. Munz C. Distinct memory CD4+ T-cell subsets mediate immune recognition of Epstein Barr virus nuclear antigen 1 in healthy virus carriers. Blood. 2007;109:1138–1146. doi: 10.1182/blood-2006-05-023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hikono H. Kohlmeier JE. Takamura S. Wittmer ST. Roberts AD. Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hildeman D. Yanez D. Pederson K. Havighurst T. Muller D. Vaccination against persistent viral infection exacerbates CD4+ T-cell-mediated immunopathological disease. J Virol. 1997;71:9672–9678. doi: 10.1128/jvi.71.12.9672-9678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hogan RJ. Cauley LS. Ely KH, et al. Long-term maintenance of virus-specific effector memory CD8+ T cells in the lung airways depends on proliferation. J Immunol. 2002;169:4976–4981. doi: 10.4049/jimmunol.169.9.4976. [DOI] [PubMed] [Google Scholar]
- 56.Hogan RJ. Usherwood EJ. Zhong W. Roberts AA. Dutton RW. Harmsen AG. Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 57.Hogan RJ. Zhong W. Usherwood EJ. Cookenham T. Roberts AD. Woodland D. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Humphreys IR. Walzl G. Edwards L. Rae A. Hill S. Hussell T. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J Exp Med. 2003;198:1237–1242. doi: 10.1084/jem.20030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jelley-Gibbs DM. Brown DM. Dibble JP. Haynes L. Eaton SM. Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaech SM. Wherry EJ. Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 61.Kaiserlian D. Cerf-Bensussan N. Hosmalin A. The mucosal immune system: from control of inflammation to protection against infections. J Leukoc Biol. 2005;78:311–318. doi: 10.1189/jlb.0105053. [DOI] [PubMed] [Google Scholar]
- 62.Kassiotis G. Gray D. Kiafard Z. Zwirner J. Stockinger B. Functional specialization of memory Th cells revealed by expression of integrin CD49b. J Immunol. 2006;177:968–975. doi: 10.4049/jimmunol.177.2.968. [DOI] [PubMed] [Google Scholar]
- 63.Kedzierska K. Stambas J. Jenkins MR. Keating R. Turner SJ. Doherty PC. Location rather than CD62L phenotype is critical in the early establishment of influenza-specific CD8+ T cell memory. Proc Natl Acad Sci USA. 2007;104:9782–9787. doi: 10.1073/pnas.0703699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim SK. Schluns KS. Lefrancois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J Immunol. 1999;163:4125–4132. [PubMed] [Google Scholar]
- 65.Kivisakk P. Mahad DJ. Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci USA. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klonowski KD. Marzo AL. Williams KJ. Lee SJ. Pham QM. Lefrancois L. CD8 T cell recall responses are regulated by the tissue tropism of the memory cell and pathogen. J Immunol. 2006;177:6738–6746. doi: 10.4049/jimmunol.177.10.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klonowski KD. Williams KJ. Marzo AL. Blair DA. Lingenheld EG. Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi N. Kondo T. Takata H. Yokota S. Takiguchi M. Functional and phenotypic analysis of human memory CD8+ T cells expressing CXCR3. J Leukoc Biol. 2006;80:320–329. doi: 10.1189/jlb.1205725. [DOI] [PubMed] [Google Scholar]
- 69.Krawczyk CM. Shen H. Pearce EJ. Functional plasticity in memory T helper cell responses. J Immunol. 2007;178:4080–4088. doi: 10.4049/jimmunol.178.7.4080. [DOI] [PubMed] [Google Scholar]
- 70.Kunkel EJ. Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 71.Kyoizumi S. Ohara T. Kusunoki Y. Hayashi T. Koyama K. Tsuyama N. Expression characteristics and stimulatory functions of CD43 in human CD4+ memory T cells: analysis using a monoclonal antibody to CD43 that has a novel lineage specificity. J Immunol. 2004;172:7246–7253. doi: 10.4049/jimmunol.172.12.7246. [DOI] [PubMed] [Google Scholar]
- 72.Lanzavecchia A. Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 73.Lee WT. Yin X-M. Vitetta ES. Functional and onto-genetic analysis of murine CD45Rhi and CD45Rlo CD4+ T cells. J Immunol. 1990;l144:3288–3295. [PubMed] [Google Scholar]
- 74.Lefrancois L. Marzo AL. The descent of memory T-cell subsets. Nat Rev Immunol. 2006;6:618–623. doi: 10.1038/nri1866. [DOI] [PubMed] [Google Scholar]
- 75.Lefrancois L. Masopust D. T cell immunity in lymphoid and non-lymphoid tissues. Curr Opin Immunol. 2002;14:503–508. doi: 10.1016/s0952-7915(02)00360-6. [DOI] [PubMed] [Google Scholar]
- 76.Li Q. Duan L. Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 77.Linsley PS. Wallace PM. Johnson J, et al. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 78.Liu F. Feuer R. Hassett DE. Whitton JL. Peptide vaccination of mice immune to LCMV or vaccinia virus causes serious CD8 T cell-mediated, TNF-dependent immunopathology. J Clin Invest. 2006;116:465–475. doi: 10.1172/JCI25608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.London CA. Lodge MP. Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 80.Lozza L. Rivino L. Guarda G, et al. The strength of T cell stimulation determines IL-7 responsiveness, secondary expansion, and lineage commitment of primed human CD4(+)IL-7R(hi) T cells. Eur J Immunol. 2008;38:30–39. doi: 10.1002/eji.200737852. [DOI] [PubMed] [Google Scholar]
- 81.Makedonas G. Betts MR. Polyfunctional analysis of human T cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin Immunopathol. 2006;28:209–219. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 82.Marshall DR. Turner SJ. Belz GT, et al. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci USA. 2001;98:6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marzo AL. Klonowski KD. Le Bon A. Borrow P. Tough DF. Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marzo AL. Yagita H. Lefrancois L. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J Immunol. 2007;179:36–40. doi: 10.4049/jimmunol.179.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masopust D. Lefrancois L. CD8 T-cell memory: the other half of the story. Microbes Infect. 2003;5:221–226. doi: 10.1016/s1286-4579(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 86.Masopust D. Vezys V. Marzo AL. Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 87.Masopust D. Vezys V. Usherwood EJ, et al. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 88.Masopust D. Vezys V. Wherry EJ. Barber DL. Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 89.McNeal MM. VanCott JL. Choi AH, et al. CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after intranasal immunization with a chimeric VP6 protein and the adjuvant LT(R192G) J Virol. 2002;76:560–568. doi: 10.1128/JVI.76.2.560-568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Messi M. Giacchetto I. Nagata K. Lanzavecchia A. Natoli G. Sallusto F. Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nat Immunol. 2003;4:78–86. doi: 10.1038/ni872. [DOI] [PubMed] [Google Scholar]
- 91.Mirenda V. Jarmin SJ. David R, et al. Physiologic and aberrant regulation of memory T-cell trafficking by the costimulatory molecule CD28. Blood. 2007;109:2968–2977. doi: 10.1182/blood-2006-10-050724. [DOI] [PubMed] [Google Scholar]
- 92.Moon JJ. Chu HH. Pepper M. McSorley SJ. Jameson SC. Kedl RM. Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mosmann TR. Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 94.Moulton VR. Bushar ND. Leeser DB. Patke DS. Farber DL. Divergent generation of heterogeneous memory CD4 T cells. J Immunol. 2006;177:869–876. doi: 10.4049/jimmunol.177.2.869. [DOI] [PubMed] [Google Scholar]
- 95.Moulton VR. Farber DL. Committed to memory: lineage choices for activated T cells. Trends Immunol. 2006;27:261–267. doi: 10.1016/j.it.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 96.Moyron-Quiroz JE. Rangel-Moreno J. Hartson L, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 97.Ndejembi MP. Tang AL. Farber DL. Reshaping the past: Strategies for modulating T-cell memory immune responses. Clin Immunol. 2007;122:1–12. doi: 10.1016/j.clim.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 98.Ndejembi MP. Teijaro JR. Patke DS, et al. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177:7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 99.Neal ZC. Splitter GA. Picornavirus-specific CD4+ T lymphocytes possessing cytolytic activity confer protection in the absence of prophylactic antibodies. J Virol. 1995;69:4914–4923. doi: 10.1128/jvi.69.8.4914-4923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohara T. Koyama K. Kusunoki Y. Hayashi T. Tsuyama N. Kubo Y. Kyoizumi S. Memory functions and death proneness in three CD4+CD45RO+ human T cell subsets. J Immunol. 2000;169:39–48. doi: 10.4049/jimmunol.169.1.39. [DOI] [PubMed] [Google Scholar]
- 101.Okoye A. Meier-Schellersheim M. Brenchley JM, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ostler T. Hussell T. Surh CD. Openshaw P. Ehl S. Long-term persistence and reactivation of T cell memory in the lung of mice infected with respiratory syncytial virus. Eur J Immunol. 2001;31:2574–2582. doi: 10.1002/1521-4141(200109)31:9<2574::aid-immu2574>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 103.Pahar B. Lackner AA. Veazey RS. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur J Immunol. 2006;36:583–592. doi: 10.1002/eji.200535520. [DOI] [PubMed] [Google Scholar]
- 104.Pantaleo G. Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat Rev Immunol. 2006;6:417–423. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
- 105.Parretta E. Cassese G. Barba P. Santoni A. Guardiola J. Di Rosa F. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J Immunol. 2005;174:7654–7664. doi: 10.4049/jimmunol.174.12.7654. [DOI] [PubMed] [Google Scholar]
- 106.Patke DS. Farber DL. Modulation of memory CD4 T cell function and survival potential by altering the strength of the recall stimulus. J Immunol. 2005;174:5433–5443. doi: 10.4049/jimmunol.174.9.5433. [DOI] [PubMed] [Google Scholar]
- 107.Perarnau B. Saron MF. San Martin BR, et al. Single H2Kb, H2Db and double H2KbDb knockout mice: peripheral CD8+ T cell repertoire and anti-lymphocytic choriomeningitis virus cytolytic responses. Eur J Immunol. 1999;29:1243–1252. doi: 10.1002/(SICI)1521-4141(199904)29:04<1243::AID-IMMU1243>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 108.Pober JS. Kluger MS. Schechner JS. Human endothelial cell presentation of antigen and the homing of memory/effector T cells to skin. Ann NY Acad Sci. 2001;941:12–25. doi: 10.1111/j.1749-6632.2001.tb03706.x. [DOI] [PubMed] [Google Scholar]
- 109.Pourgheysari B. Khan N. Best D. Bruton R. Nayak L. Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol. 2007;81:7759–7765. doi: 10.1128/JVI.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Richards H. Longhi MP. Wright K. Gallimore A. Ager A. CD62L (L-selectin) down-regulation does not affect memory T cell distribution but failure to shed compromises anti-viral immunity. J Immunol. 2008;180:198–206. doi: 10.4049/jimmunol.180.1.198. [DOI] [PubMed] [Google Scholar]
- 111.Rivino L. Messi M. Jarrossay D. Lanzavecchia A. Sallusto F. Geginat J. Chemokine receptor expression identifies pre-T helper (Th)1, pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med. 2004;200:725–735. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roberts AD. Ely KH. Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roberts AD. Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J Immunol. 2004;172:6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 114.Rogers PR. Dubey C. Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 115.Romero P. Zippelius A. Kurth I, et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 116.Rose JR. Williams MB. Rott LS. Butcher EC. Greenberg HB. Expression of the mucosal homing receptor alpha4beta7 correlates with the ability of CD8+ memory T cells to clear rotavirus infection. J Virol. 1998;72:726–730. doi: 10.1128/jvi.72.1.726-730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rothoeft T. Fischer K. Zawatzki S. Schulz V. Schauer U. Korner Rettberg C. Differential response of human naive and memory/effector T cells to dendritic cells infected by respiratory syncytial virus. Clin Exp Immunol. 2007;150:263–273. doi: 10.1111/j.1365-2249.2007.03497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sallusto F. Geginat J. Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 119.Sallusto F. Langenkamp A. Geginat J. Lanzavecchia A. Functional subsets of memory T cells identified by CCR7 expression. Curr Top Microbiol Immunol. 2000;251:167–171. doi: 10.1007/978-3-642-57276-0_21. [DOI] [PubMed] [Google Scholar]
- 120.Sallusto F. Lenig D. Forster R. Lipp M. Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions [see comments] Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 121.Selin LK. Brehm MA. Naumov YN. Cornberg M. Kim SK. Clute SC. Welsh RM. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Selin LK. Nahill SR. Welsh RM. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Selin LK. Varga SM. Wong IC. Welsh RM. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med. 1998;188:1705–1715. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Selin LK. Welsh RM. Plasticity of T cell memory responses to viruses. Immunity. 2004;20:5–16. doi: 10.1016/S1074-7613(03)00356-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shiao SL. McNiff JM. Pober JS. Memory T cells and their costimulators in human allograft injury. J Immunol. 2005;175:4886–4896. doi: 10.4049/jimmunol.175.8.4886. [DOI] [PubMed] [Google Scholar]
- 126.Singh A. Suresh M. A role for TNF in limiting the duration of CTL effector phase and magnitude of CD8 T cell memory. J Leukoc Biol. 2007;82:1201–1211. doi: 10.1189/jlb.0407240. [DOI] [PubMed] [Google Scholar]
- 127.Slifka MK. Whitton JL. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J Immunol. 2000;164:208–216. doi: 10.4049/jimmunol.164.1.208. [DOI] [PubMed] [Google Scholar]
- 128.Smiley KL. McNeal MM. Basu M. Choi AH. Clements JD. Ward RL. Association of gamma interferon and interleukin-17 production in intestinal CD4+ T cells with protection against rotavirus shedding in mice intranasally immunized with VP6 and the adjuvant LT(R192G) J Virol. 2007;81:3740–3748. doi: 10.1128/JVI.01877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smythies LE. Sellers M. Clements RH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Soler D. Chapman TR. Poisson LR, et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J Immunol. 2006;177:6940–6951. doi: 10.4049/jimmunol.177.10.6940. [DOI] [PubMed] [Google Scholar]
- 131.Song K. Rabin RL. Hill BJ, et al. Characterization of subsets of CD4+ memory T cells reveals early branched pathways of T cell differentiation in humans. Proc Natl Acad Sci USA. 2005;102:7916–7921. doi: 10.1073/pnas.0409720102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sprent J. Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–579. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 133.Stohlman SA. Hinton DR. Parra B. Atkinson R. Bergmann CC. CD4 T cells contribute to virus control and pathology following CNS infection by neurotropic mouse hepatitis virus. J Virol. 2008;82:2130–2139. doi: 10.1128/JVI.01762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stubbe M. Vanderheyde N. Goldman M. Marchant A. Antigen-specific central memory CD4+ T lymphocytes produce multiple cytokines and proliferate in vivo in humans. J Immunol. 2006;177:8185–8190. doi: 10.4049/jimmunol.177.11.8185. [DOI] [PubMed] [Google Scholar]
- 135.Stubbe M. Vanderheyde N. Pircher H. Goldman M. Marchant A. Characterization of a subset of antigen-specific human central memory CD4+ T lymphocytes producing effector cytokines. Eur J Immunol. 2008;38:273–282. doi: 10.1002/eji.200737611. [DOI] [PubMed] [Google Scholar]
- 136.Sun Y. Schmitz JE. Buzby AP, et al. Virus-specific cellular immune correlates of survival in vaccinated monkeys after simian immunodeficiency virus challenge. J Virol. 2006;80:10950–10956. doi: 10.1128/JVI.01458-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Swain SL. Dutton RW. Woodland DL. T cell responses to influenza virus infection: effector and memory cells. Viral Immunol. 2004;17:197–209. doi: 10.1089/0882824041310577. [DOI] [PubMed] [Google Scholar]
- 138.Takata H. Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol. 2006;177:4330–4340. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- 139.Unsoeld H. Krautwald S. Voehringer D. Kunzendorf U. Pircher H. Cutting edge: CCR7+ and CCR7− memory T cells do not differ in immediate effector cell function. J Immunol. 2002;169:638–641. doi: 10.4049/jimmunol.169.2.638. [DOI] [PubMed] [Google Scholar]
- 140.Unsoeld H. Pircher H. Complex memory T-cell phenotypes revealed by coexpression of CD62L and CCR7. J Virol. 2005;79:4510–4513. doi: 10.1128/JVI.79.7.4510-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Usherwood EJ. Hogan RJ. Crowther G. Surman SL. Hogg TL. Altman JD. Woodland DL. Functionally heterogeneous CD8(+) T-cell memory is induced by Sendai virus infection of mice. J Virol. 1999;73:7278–7286. doi: 10.1128/jvi.73.9.7278-7286.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Valujskikh A. Pantenburg B. Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 143.van Faassen H. Saldanha M. Gilbertson D. Dudani R. Krishnan L. Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J Immunol. 2005;174:5341–5350. doi: 10.4049/jimmunol.174.9.5341. [DOI] [PubMed] [Google Scholar]
- 144.Veazey RS. Tham IC. Mansfield KG, et al. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Verhoeven D. Sankaran S. Dandekar S. Simian immunodeficiency virus infection induces severe loss of intestinal central memory T cells which impairs CD4+ T-cell restoration during antiretroviral therapy. J Med Primatol. 2007;36:219–227. doi: 10.1111/j.1600-0684.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 146.Verhoeven D. Sankaran S. Silvey M. Dandekar S. Antiviral therapy during primary SIV infection fails to prevent acute CD4+ T-cell loss in gut mucosa but enhances their rapid restoration through central memory T-cells. J Virol. 2008. in press. [DOI] [PMC free article] [PubMed]
- 147.Wallace DL. Beverley PCL. Phenotypic changes associated with activation of CD45RA+ and CD45RO+ T cells. Immunology. 1990;69:460–467. [PMC free article] [PubMed] [Google Scholar]
- 148.Weaver CT. Harrington LE. Mangan PR. Gavrieli M. Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 149.Weaver CT. Hatton RD. Mangan PR. Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 150.Weinberger B. Lazuardi L. Weiskirchner I, et al. Healthy aging and latent infection with CMV lead to distinct changes in CD8+ and CD4+ T-cell subsets in the elderly. Hum Immunol. 2007;68:86–90. doi: 10.1016/j.humimm.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 151.Welsh RM. McNally JM. Brehm MA. Selin LK. Consequences of cross-reactive and bystander CTL responses during viral infections. Virology. 2000;270:4–8. doi: 10.1006/viro.2000.0278. [DOI] [PubMed] [Google Scholar]
- 152.Welsh RM. Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 153.Westendorf AM. Templin M. Geffers R, et al. CD4+ T cell mediated intestinal immunity: chronic inflammation versus immune regulation. Gut. 2005;54:60–69. doi: 10.1136/gut.2003.037663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wherry EJ. Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wherry EJ. Teichgraber V. Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 156.Williams MA. Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 157.Woodland DL. Immunologic memory. Viral Immunol. 2007;20:229–230. doi: 10.1089/vim.2007.ED20.2. [DOI] [PubMed] [Google Scholar]
- 158.Woodland DL. Hogan RJ. Zhong W. Cellular immunity and memory to respiratory virus infections. Immunol Res. 2001;24:53–67. doi: 10.1385/IR:24:1:53. [DOI] [PubMed] [Google Scholar]
- 159.Woodland DL. Scott I. T cell memory in the lung airways. Proc Am Thorac Soc. 2005;2:126–131. doi: 10.1513/pats.200501-003AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu L. Yoon H. Zhao MQ. Liu J. Ramana CV. Enelow RI. Cutting edge: pulmonary immunopathology mediated by antigen-specific expression of TNF-alpha by antiviral CD8+ T cells. J Immunol. 2004;173:721–725. doi: 10.4049/jimmunol.173.2.721. [DOI] [PubMed] [Google Scholar]
- 161.Xu RH. Fang M. Klein-Szanto A. Sigal LJ. Memory CD8+ T cells are gatekeepers of the lymph node draining the site of viral infection. Proc Natl Acad Sci USA. 2007;104:10992–10997. doi: 10.1073/pnas.0701822104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yamaguchi Y. Fujio K. Shoda H. Okamoto A. Tsuno NH. Takahashi K. Yamamoto K. IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. J Immunol. 2007;179:7128–7136. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- 163.Yang J. Brook MO. Carvalho-Gaspar M, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci USA. 2007;104:19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang L. Peng H. Zhu Z, et al. Persistent memory CD4+ and CD8+ T-cell responses in recovered severe acute respiratory syndrome (SARS) patients to SARS coronavirus M antigen. J Gen Virol. 2007;88:2740–2748. doi: 10.1099/vir.0.82839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yuen KY. Chan PK. Peiris M, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 166.Zammit DJ. Cauley LS. Pham QM. Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zammit DJ. Turner DL. Klonowski KD. Lefrancois L. Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhai Y. Meng L. Gao F. Busuttil RW. Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169:4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 169.Zhang X. Dong H. Lin W, et al. Human bone marrow: a reservoir for “enhanced effector memory” CD8+ T cells with potent recall function. J Immunol. 2006;177:6730–6737. doi: 10.4049/jimmunol.177.10.6730. [DOI] [PubMed] [Google Scholar]


