Table 1.
Inhibitory Activity of Compounds against Wild-Type HIV-1 Protease and an MDR Varianta
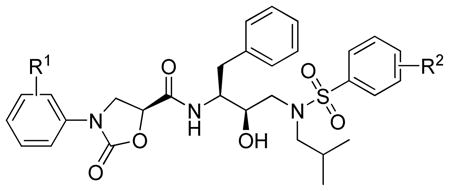 | ||||
|---|---|---|---|---|
| Compd. | R1 | R2 |
Ki (nM) |
|
| Wt | MDR | |||
| 15c | H | 3,4-OCH2O- | 0.206 | 5.50 |
| 15d | H | 3,4-S-C=N- | 0.033 | 1.21 |
| 15e | H | 4-CH2OH | 0.252 | NT |
| 17a | 2-OH | 4-NH2 | 7.36 | 40.46 |
| 17b | 2-OH | 4-OCH3 | 5.65 | 67.34 |
| 17c | 2-OH | 3,4-OCH2O- | 1.47 | NT |
| 18a | 2-CF3 | 4-NH2 | 0.136 | 5.71 |
| 18b | 2-CF3 | 4-OCH3 | 0.086 | 2.45 |
| 18c | 2-CF3 | 3,4-OCH2O- | 0.142 | 4.36 |
| 18d | 2-CF3 | 3,4-S-C=N- | 0.097 | 1.86 |
| 18e | 2-CF3 | 4-CH2OH | 0.235 | 4.57 |
| 19a | 2,4-di-F | 4-NH2 | 0.385 | NT |
| 19b | 2,4-di-F | 4-OCH3 | 0.063 | 10.54 |
| 19c | 2,4-di-F | 3,4-OCH2O- | 0.347 | NT |
| 19d | 2,4-di-F | 3,4-S-C=N- | 0.150 | 3.50 |
| 19e | 2,4-di-F | 4-CH2OH | 0.212 | NT |
| 20a | 4-F | 4-NH2 | 0.448 | NT |
| 20b | 4-F | 4-OCH3 | 0.128 | 9.57 |
| 20c | 4-F | 3,4-OCH2O- | 0.167 | 10.78 |
| 20d | 4-F | 3,4-S-C=N- | 0.133 | 3.0 |
| 20e | 4-F | 4-CH2OH | 0.207 | 9.56 |
| 21d | 4-Ac | 3,4-S-C=N- | 0.073 | 2.04 |
| 21e | 4-Ac | 4-CH2OH | 0.317 | NT |
| 22d | 3-F | 3,4-S-C=N- | 0.080 | 2.12 |
| 22e | 3-F | 4-CH2OH | 0.319 | NT |
| 23d | 3,4-di-F | 3,4-S-C=N- | 0.232 | 5.18 |
| 23e | 3,4-di-F | 4-CH2OH | 0.330 | NT |
| 24d | 3-CF3 | 3,4-S-C=N- | 0.016 | 2.96 |
| 24e | 3-CF3 | 4-CH2OH | 0.196 | 10.15 |
| 25a | 3-OCF3 | 4-NH2 | 0.225 | 9.95 |
| 25b | 3-OCF3 | 4-OCH3 | 0.130 | 16.3 |
| 25c | 3-OCF3 | 3,4-OCH2O- | 0.239 | 10.6 |
| 25d | 3-OCF3 | 3,4-S-C=N- | 0.026 | 3.38 |
| 25e | 3-OCF3 | 4-CH2OH | 0.286 | 7.49 |
| 26d | 3-Ac | 3,4-S-C=N- | 0.015 | 1.69 |
| 26e | 3-Ac | 4-CH2OH | 0.236 | NT |
| 27b | 3-SO2CH3 | 4-OCH3 | 0.049 | 1.74 |
| 27c | 3-SO2CH3 | 3,4-OCH2O- | 0.025 | 4.16 |
| 27d | 3-SO2CH3 | 3,4-S-C=N- | 0.003 | 2.45 |
| 28b | 3-NO2 | 4-OCH3 | 0.136 | 5.40 |
| 28c | 3-NO2 | 3,4-OCH2O- | 0.117 | 6.48 |
| 28d | 3-NO2 | 3,4-S-C=N- | 0.015 | 0.93 |
| 29b | 3-NH2 | 4-OCH3 | 0.020 | 2.68 |
| 29c | 3-NH2 | 3,4-OCH2O- | 0.113 | 6.55 |
| 29d | 3-NH2 | 3,4-S-C=N- | 0.008 | 1.84 |
| 30b | 3-NHAc | 4-OCH3 | 0.122 | 3.89 |
| 30c | 3-NHAc | 3,4-OCH2O- | 0.051 | 4.76 |
| 30d | 3-NHAc | 3,4-S-C=N- | 0.006 | 2.83 |
| 31b | 3-NHCO2CH3 | 4-OCH3 | 0.163 | 4.44 |
| 31c | 3-NHCO2CH3 | 3,4-OCH2O- | 0.289 | 3.68 |
| 31d | 3-NHCO2CH3 | 3,4-S-C=N- | 0.026 | 1.87 |
| LPV | 0.005 | 0.90 | ||
| DRV | 0.008 | 0.025 | ||
Wt: Q7K; MDR: L10I, L63P, A71V, G73S, I84V, L90M. NT = not tested
