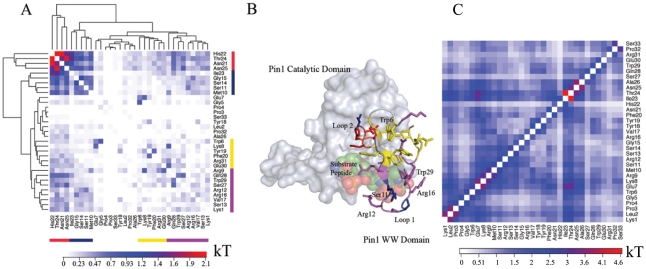
Figure 1. Correlated motions couple the catalytic domain interface to the substrate-binding loop of Pin1's WW domain.
The WW domain is shown in cartoon and sticks, the catalytic domain as a surface, and the substrate in spheres. The structure shown is from PDB entry 1F8A. Only the WW domain was simulated; the catalytic domain is only shown for reference. (A) Hierarchical clustering of the mutual information between residues' torsions identifies several functionally important groups of residues. (B) Most residues in the red cluster lie in the catalytic domain interface and are correlated with residues in magenta cluster, which includes a number of key substrate-binding residues. All residues exhibiting slow motions in NMR experiments are in either the red or magenta clusters. (C) Mutual information between  atoms complements torsional analysis and importantly captures correlated motions of secondary structure elements, highlighting correlated motions between the first
atoms complements torsional analysis and importantly captures correlated motions of secondary structure elements, highlighting correlated motions between the first  (residues 7–9) and Loop 1 (residues 10–16), between the first
(residues 7–9) and Loop 1 (residues 10–16), between the first  and the second
and the second  (residues 17–21), and between the C-terminal part of Loop 2 and the beginning of the third
(residues 17–21), and between the C-terminal part of Loop 2 and the beginning of the third  (residues 23–26) and the rest of the protein.
(residues 23–26) and the rest of the protein.