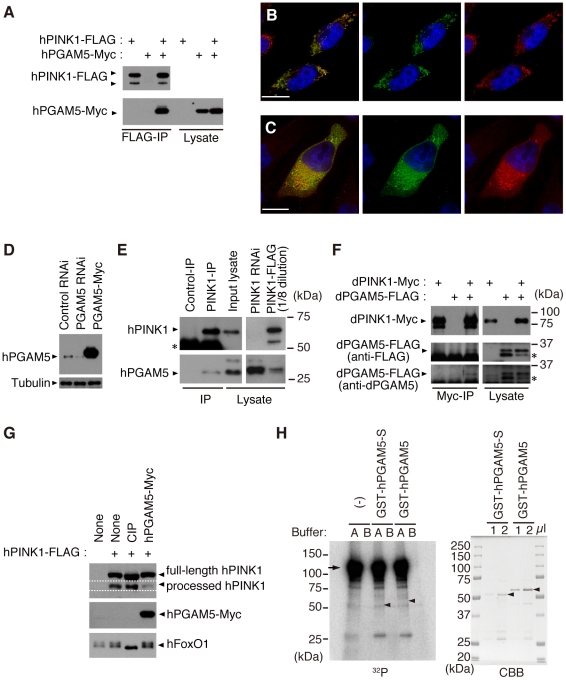
Figure 2. PGAM5 associates with PINK1 at mitochondria.
(A) hPGAM5 binds to hPINK1 in HEK293 cells. Lysate expressing C-terminally Myc-tagged hPGAM5 (hPGAM5-Myc) and FLAG-tagged hPINK1 (hPINK1-FLAG) was subjected to immunoprecipitation with anti-FLAG antibody (FLAG-IP), and analyzed by immunoblotting with anti-tag antibodies. (B) hPGAM5 is localized to the mitochondria. HeLa cells transfected with hPGAM5-Myc were visualized with anti-Myc (green). Mitochondria were visualized with MitoTracker (red) and nuclei with DAPI (blue). Regions of co-localization of hPGAM5 with mitochondria appear in yellow in the merged image. (C) hPGAM5 and hPINK1 co-localize at mitochondria. HeLa cells co-transfected with hPINK1 and hPGAM5-Myc were stained with anti-PINK1 (green) and anti-Myc (red). (D) Anti-hPGAM5 antibody specifically recognizes ∼30 kDa bands in extract from HEK293 cells, which were reduced in lysates from cells treated with siRNAs directed against hPGAM5. Lysate expressing hPGAM5-Myc and anti-tubulin signals served as a positive control and a loading control, respectively. (E) Endogenous hPGAM5 is associated with hPINK1. An anti-PINK1 (PINK1-IP) or an antibody against the unrelated protein Delta (Control-IP) was used for immunoprecipitation of proteins in HEK293 cells. Cell lysate in which hPINK1 was knocked down by RNAi (PINK1 RNAi) and lysate from cells that overexpressed hPINK1-FLAG (PINK1-FLAG) served as additional controls. The PINK1-FLAG lysate was diluted eight-fold with loading buffer to reduce the strong signal present in that sample. Asterisk, bands attributable to detection of the antibodies themselves, which may mask lower molecular weight hPINK1 bands (∼52 kDa). (F) dPGAM5 is associated with dPINK1 in Drosophila S2 cells. S2 cell lysate expressing dPINK1-Myc and dPGAM5-FLAG was subjected to immunoprecipitation with anti-Myc antibody (Myc-IP), and analyzed by immunoblotting with anti-tag or anti-dPGAM5 antibodies. Asterisks, a putative processed form of dPGAM5. (G) HEK 293 cell lysate expressing hPINK1-FLAG together with hPGAM5-Myc was subjected to Phos-tag immunoblotting [43]. hPINK1-FLAG lysate treated with alkaline phosphatase (CIP) was used as a positive control. A phospho-protein FoxO1 was efficiently dephosphorylated by the CIP treatment. (H) An in vitro kinase assay was performed using 2x GST-dPINK1 and GST-hPGAM5. Recombinant 2x GST-dPINK1 purified from bacteria was used as a kinase source. Recombinant GST-hPGAM5 short form (GST-hPGAM5-S) or GST-hPGAM5 was purified from bacteria and 1 and 2 µl of the purified fractions were separated by SDS-PAGE and stained with Coomassie Brilliant Blue (CBB, right-hand panel; arrowheads, GST-hPGAM5-S or GST-hPGAM5). A total of 100 or 400 ng of GST-hPGAM5-S or GST-hPGAM5, respectively, were incubated with 100 ng of 2x GST-dPINK1 in kinase reaction buffer A (100 mM Tris-HCl [pH 7.5], 240 mM NaCl, 30 µM ATP, 10 mM MgCl2, 2 mM CaCl2, 5 µCi γ-32P ATP) or buffer B (100 mM Tris-HCl [pH7.5], 240 mM NaCl, 30 µM ATP, 10 mM EDTA, 5 µCi γ-32P ATP) for 30 min at 30°C. The reaction mixture was suspended in SDS sample buffer and then subjected to SDS-PAGE and autoradiography (Left, 32P; the arrow and arrowheads represent expected migration positions of 2x GST-dPINK1 and GST-hPGAM5/GST-hPGAM5-S, respectively). No specific signals corresponding hPGAM5 or hPGAM5-S were observed. Note that 2x GST-dPINK1 lacks kinase activity in the buffer B, suggesting that activation of PINK1 requires divalent cations such as Mg2+ and Ca2+. Scale bars = 15 µm in (B and C).