Table 2.
One-pot synthesis of allylic amines using an organocatalytic aziridination-Wharton sequence 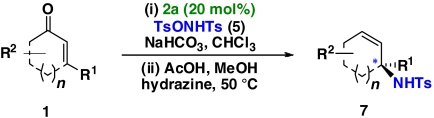
| Entry |
Enone (1) |
Product (7) |
Yield (%)* |
ee (%)† |
||
| 1 | 1a | 7a | 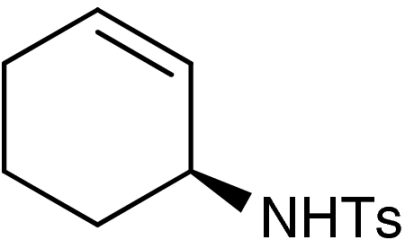 |
56 | 96 | |
| 2‡ | 1a | ent-7a | 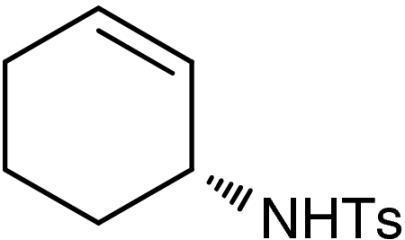 |
52 | 95 | |
| 3 | 1b | 7b | 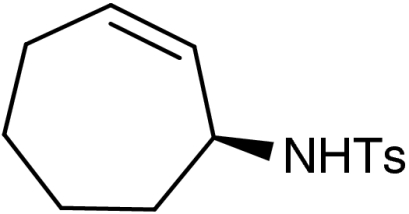 |
48 (37)§ | 98 (98)§ | |
| 4 | 1d | 7d | 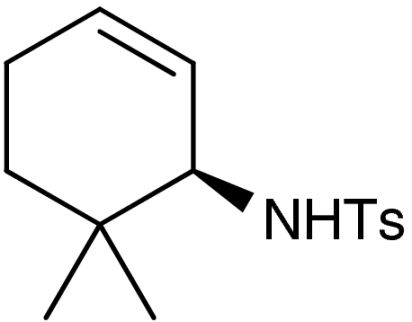 |
50 (50)§ | 97 (97)§ | |
| 5 | 1e | 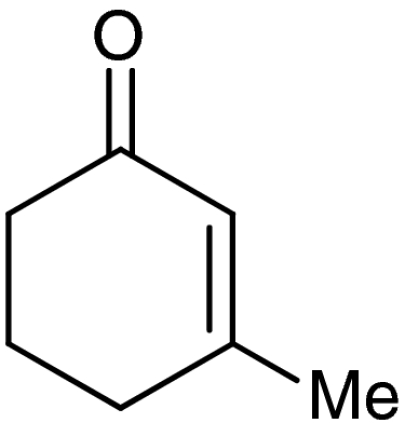 |
7e | 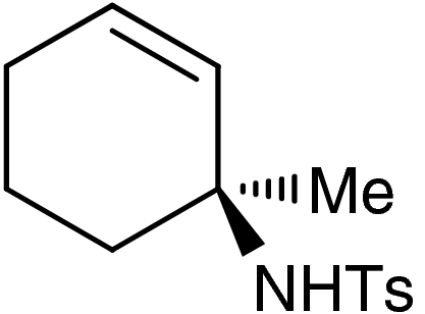 |
57 | 99 |
| 6 | 1f | 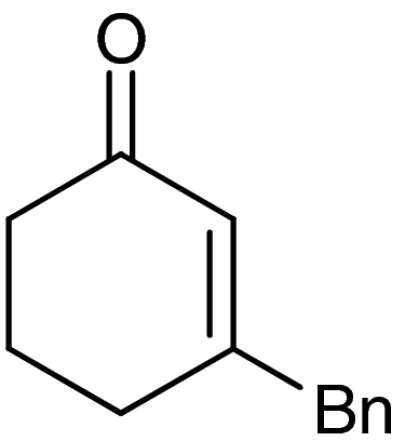 |
7f | 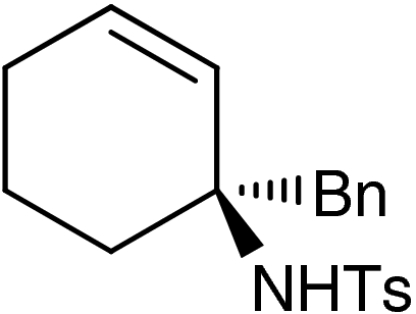 |
61 | 99 |
| 7‡ | 1f | 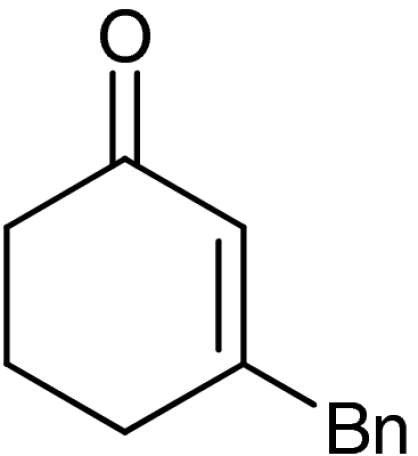 |
ent-7f | 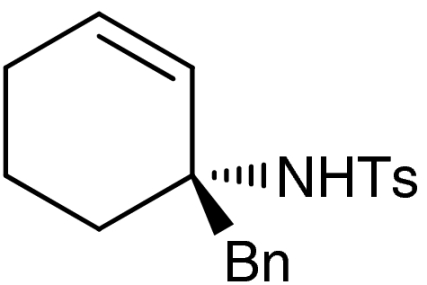 |
69 | 99 |
| 8 | 1g | 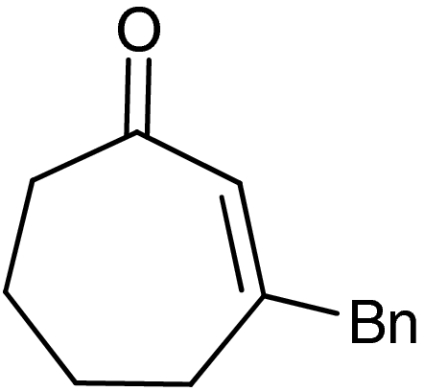 |
7g | 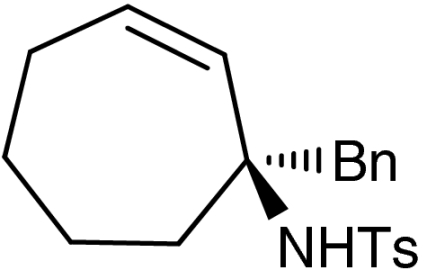 |
42 | 94 |
| 9 | 1h | 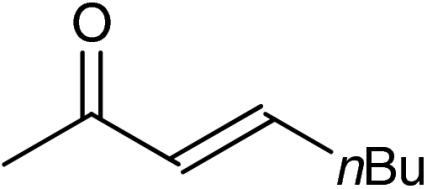 |
7h | 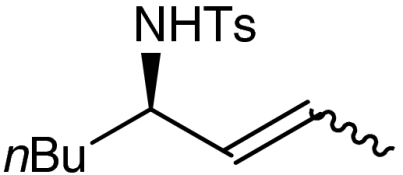 |
52 | 97 |
*Yields of isolated products.
†Determined by chiral stationary phase HPLC.
‡The quasi-enantiomer of the catalyst was used (2b, see Scheme 5).
§Results for the sequence performed in two separate steps.
