Table 1.
Conditions for catalytically generation of enolate equivalents from cinnamaldehyde for hetero-Diels–Alder reactions* 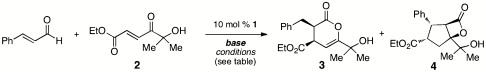
| Entry | Conditions | Base | Ratio 3∶4† |
| 1 | 0.1 M THF, RT | 25% DBU | 1∶1 |
| 2 | 0.1 M EtOH, RT | 25% DBU | No annulation products |
| 3 | 0.1 M DCE, RT | 25% DBU | 1∶1 |
| 0.1 M Tol, RT | 25% DBU | 1∶5 | |
| 8 | 25% DIPEA | 1∶0 (side products observed)‡ | |
| 9 | 25% DMAP | 1∶0 | |
| 10 | 1 equivalent DBU | 0∶1 | |
| 1.5 equivalent NEt3 | 1∶5 | ||
| 11 | 0.1 M Tol, 40 °C | 25% DBU | 1∶10 |
| 12 | 0.1 M CH2Cl2, 40 °C | 15% DMAP | 1∶0 (10∶1 d.r., 99% ee) |
| 13 | 15% NMM | 1∶0 (> 20∶1 d.r., 99% ee) |
*Reaction time: 18–24 h. DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; DMAP, N,N-4-dimethylaminopyridine; NMM, N-methylmorpholine; DIPEA. diisopropylethylamine RT, room temperature; d.r., diastereomeric ratio; ee, enantiomeric excess.
†Product ratios determined by 1H NMR of unpurified reaction mixtures. Minor cyclopentane-derived isomers also observed, see refs. 14 and 15 for details.
‡Side products include substrate decomposition or unidentified materials.
