Table 1.
Optimization of Michael–Henry reaction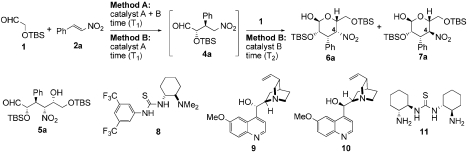
| Entry | Catalyst A | Catalyst B (mol%) | Time (T1 + T2) | Method * | Yield (%) † | dr ‡ (5a + 6a∶7a) | ee (%) § |
| 1 | 3 | Et3N (20) | 1 d | A | 62 | 7∶1 | 88 |
| 2 | 3 | 8 (20) | 1 d | A | 54 | 4∶1 | 89 |
| 3 | 3 | 9 (20) | 1 d | A | 62 | 6∶1 | 87 |
| 4 | 3 | 10 (20) | 1 d | A | 58 | 6∶1 | 87 |
| 5 | - | Et3N (20) | 1 d | A | 27 | 3∶1 | — |
| 6 | - | 8 (20) | 1 d | A | 17 | 3∶1 | -35 |
| 7 | 3 | Et3N (50) | 4 + 1 h | B | 68 | > 10∶1 | 98 |
| 8 | 3 | i Pr 2EtN (50) | 4 + 1 h | B | 68 | > 10∶1 | 98 |
| 9 | 3 | DABCO (25) | 4 + 1 h | B | 44 | 4∶1 | 98 |
| 10 | 3 | DMAP (50) | 4 + 1 h | B | 15 | 1∶1 | 98 |
| 11 | 3 | DBU (50) | 4 + 1 h | B | 51 | 0∶1 | 98 |
| 12 ¶ | 11 ∥ | Et3N (50) | 8 + 2 h | B | 38 | 8∶1 | 96 |
*Method A: catalyst A (20 mol%), catalyst B and 2a (0.1 or 0.2 mmol) were reacted with 1 (4 equiv.) in CH2Cl2 at rt for T1. Method B: catalyst A (20 mol%) and 2a (0.1 or 0.2 mmol) were reacted with 1 (4 equiv.) in CH2Cl2 at 30 °C for T1, then catalyst B was added (see text).
†Yield of isolated product.
‡Determined by 1H NMR analysis of purified product, (5a + 6a∶7a).
§Determined by chiral phase HPLC analysis of corresponding alcohol.
¶The reaction was carried out at rt.
∥10 mol% of 11 was used.
