Abstract
The ligand-activated transcription factor aryl hydrocarbon receptor (AHR) participates in the differentiation of FoxP3+ Treg, Tr1 cells, and IL-17–producing T cells (Th17). Most of our understanding on the role of AHR on the FoxP3+ Treg compartment results from studies using the toxic synthetic chemical 2,3,7,8-tetrachlorodibenzo-p-dioxin. Thus, the physiological relevance of AHR signaling on FoxP3+ Treg in vivo is unclear. We studied mice that carry a GFP reporter in the endogenous foxp3 locus and a mutated AHR protein with reduced affinity for its ligands, and found that AHR signaling participates in the differentiation of FoxP3+ Treg in vivo. Moreover, we found that treatment with the endogenous AHR ligand 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) given parenterally or orally induces FoxP3+ Treg that suppress experimental autoimmune encephalomyelitis. ITE acts not only on T cells, but also directly on dendritic cells to induce tolerogenic dendritic cells that support FoxP3+ Treg differentiation in a retinoic acid-dependent manner. Thus, our work demonstrates that the endogenous AHR ligand ITE promotes the induction of active immunologic tolerance by direct effects on dendritic and T cells, and identifies nontoxic endogenous AHR ligands as potential unique compounds for the treatment of autoimmune disorders.
Regulatory T cells (Treg) that express the transcription factor FoxP3 control immune autoreactivity in healthy individuals (1). FoxP3+ Treg are generated in the thymus (natural Treg, nTreg) and also in the periphery (induced Treg, iTreg). The importance of FoxP3+ Treg for immunoregulation is highlighted by the immune disorders that result from their depletion or loss of function in both mice and humans (1). Conversely, the induction of FoxP3+ Treg is viewed as a promising approach for the treatment of immune-mediated disorders (2).
We (3) and others (4–8) have found that the ligand-activated transcription factor aryl hydrocarbon receptor (AHR) controls the differentiation of Treg, Tr1 cells (9), and IL-17–producing T cells (Th17) in vitro and in vivo. AHR activation by its high-affinity ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in vivo results in the expansion of the CD4+CD25+Foxp3+ Treg compartment (3). These CD4+CD25+Foxp3+ Treg are functional and suppress the development of experimental autoimmune encephalomyelitis (EAE) (3), experimental autoimmune uveoretinitis (7), and spontaneous autoimmune diabetes (10).
TCDD is a valuable tool to study the immunological effects of AHR activation, but TCDD is not a natural AHR ligand and its toxicity rules out its therapeutic use. Thus, it is not yet known whether there is a physiological role for AHR in FoxP3+ Treg, and whether nontoxic AHR ligands exist which can expand FoxP3+ Treg in vivo to treat autoimmunity.
To address these questions, we used mice carrying a GFP reporter in foxp3 and a mutant AHR protein with reduced affinity for its ligands. In addition, we investigated the effect and mechanisms of action of the nontoxic mucosal AHR ligand 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) on FoxP3+ Treg both in vitro and in vivo in the model of EAE.
Results
AHR Activation by Endogenous Ligands Promotes the Differentiation of FoxP3+ iTreg.
FoxP3+ Treg are classified as FoxP3+ nTreg (generated in the thymus) and FoxP3+ iTreg (generated in the periphery) (1). The gut-associated lymphoid tissue is a major physiological site for the differentiation of FoxP3+ iTreg (11). To investigate the physiological role of AHR on FoxP3+ iTreg differentiation, we generated AHR-d Foxp3gfp mice and analyzed the frequency of FoxP3+ Treg in the thymus and mesenteric lymph nodes (MLN). AHR-d Foxp3gpf mice carry a GFP reporter in the foxp3 gene (12), and harbor the d allele of ahr (AHR-d), which codes for an AHR protein with reduced affinity for its ligands (13). We found a significant reduction in the frequency of FoxP3+ Treg cells in the MLN of AHR-d Foxp3gfp mice (Fig. S1A); no difference was detected in the frequency of thymic FoxP3+ Treg (Fig. S1B). WT and AHR-d Foxp3+ Treg showed comparable suppressive activity in vitro (Fig. S2).
To determine whether the reduced frequency of FoxP3+ Treg in the MLN of AHR-d Foxp3gfp mice resulted from an impaired generation of FoxP3+ iTreg in vivo, we used a transfer model (14). CD4+ FoxP3:GFP− T cells from naive AHR-d Foxp3gfp or Foxp3gfp mice were transferred to RAG-1-deficient mice, and the reconstituted mice were immunized with MOG35–55 (14). We found a significant reduction in the frequency of CD4+ FoxP3:GFP+ Treg in mice that received AHR-d FoxP3:GFP− T cells (Fig. S1C). These results suggest a physiological role for endogenous AHR ligands in the generation of FoxP3+ iTreg in vivo.
Given our results on the role of AHR on FoxP3+ iTreg in vivo, we analyzed the role of AHR on the differentiation of FoxP3+ iTreg triggered in vitro with TGF-β1 and IL-2 (3, 15). Naive CD4+ T cells from AHR-d Foxp3gfp mice showed a significant impairment in their differentiation into FoxP3+ Treg (Fig. S1D), with or without antigen presenting cells (APCs). Thus, AHR activation by endogenous ligands in T cells affects FoxP3+ iTreg differentiation.
We investigated the mechanism by which AHR signaling in T cells might influence the development of FoxP3+ iTreg. Stat1 activation interferes with the differentiation of Th17 (16) and FoxP3+ Treg (17). AHR interacts with Stat1 and limits its activation during the differentiation of Th17 cells (5). Thus, we analyzed Stat1 phosphorylation in naive CD4+ T cells from AHR-d Foxp3gfp or Foxp3gfp mice activated with TGF-β1 and IL-2 for 48 h. We found increased Stat1 phosphorylation in naive CD4+ T cells from AHR-d Foxp3gfp mice (Fig. S1E); no changes were detected in the phosphorylation of Stat5 (Fig. S3). Although an interaction between AHR and Stat3 was not found by Kimura et al. (5), we also investigated Stat3 phosphorylation in Treg and Th17 cells differentiated from naive CD4+ WT or AHR-d T cells. We did not find a significant difference in the phosphorylation of Stat3 in Treg (1.9% in WT vs. 1.1% in AHR-d). Taken together, these results suggest that AHR activation by endogenous ligands limits Stat1 signaling in T cells and promotes the differentiation of FoxP3+ iTreg.
AHR Activation by the Nontoxic Endogenous Ligand ITE Suppresses EAE.
The results above suggested that endogenous AHR ligands function in vivo to induce FoxP3+ iTreg. Several endogenous AHR ligands have been described. ITE, for example, is a nontoxic mucosal AHR ligand (18). Based on the role of AHR on the generation of FoxP3+ iTreg, and the potential of FoxP3+ Treg expansion as a therapeutic approach for the management of autoimmune disorders (2), we investigated the ability of AHR activation by ITE to induce functional FoxP3+ Treg and treat EAE.
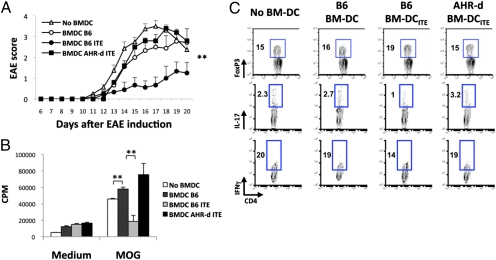
Daily ITE administration (200 μg/mice, intraperitoneally) on the day of EAE induction suppressed EAE in WT B6 mice (Fig. 1A). ITE did not suppress EAE in AHR-d mice, indicating that the effects of ITE on EAE were mediated by AHR (Fig. 1A). Oral (Fig. 1B) administration of ITE (200 μg/mice, daily) also suppressed EAE. Although the liver is highly sensitive to the toxic effects of TCDD (19), ITE administration did not result in liver toxicity (Table S1). Thus, ITE suppresses EAE by an AHR-dependent mechanism that does not involve toxicity and is active orally.
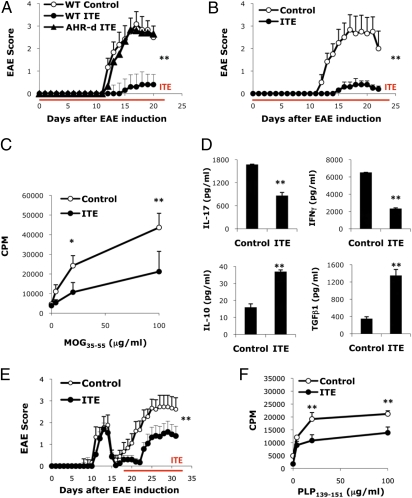
Fig. 1.
AHR activation by the nontoxic endogenous ligand ITE suppresses EAE. (A) EAE was induced in naive wild-type B6 or AHR-d mice, and ITE or vehicle as control was administered i.p. daily from the day of immunization until the termination of the experiment. The course of EAE is shown as the mean EAE score + SEM. Shown are representative data of one of three experiments that produced similar results. **P < 0.001 compared to control-treated WT mice or ITE treated AHR-d mice. (B) EAE was induced in naive wild-type mice and ITE or vehicle as control was administered orally daily from the day of immunization until the termination of the experiment. The course of EAE is shown as the mean EAE score + SEM. **P < 0.001 compared to control-treated WT mice. (C) Proliferative response to MOG35-55 of lymph node cells from ITE or control treated animals 10 d after immunization with MOG35-55 in CFA. Cell proliferation is indicated as cpm + SD in triplicate wells. *P < 0.05 and **P < 0.001 compared to cells from control-treated mice. (D) Cytokine secretion triggered by MOG35-55 in lymph node cells from ITE or control treated animals 10 d after immunization with MOG35-55 in CFA. **P < 0.001 compared to cells taken from control-treated mice. (E) EAE was induced in naive SJL mice, and ITE or vehicle as control was administered i.p. daily from day 17 after the immunization till the termination of the experiment. The course of EAE in these mice is shown as the mean EAE score + SEM. **P < 0.001 when compared to control-treated WT mice. (F) Proliferative response to PLP139-151 of splenocytes taken from ITE or control treated SJL mice 30 days after immunization with PLP139-151/CFA. Cell proliferation is indicated as cpm + SD in triplicate wells. **P < 0.001 when compared to cells taken from control-treated mice. Representative data of 1 of at least 2 experiments that produced similar results.
EAE is an autoimmune disease driven by Th1 and Th17 cells. To study the suppression of EAE by AHR activation with ITE, we analyzed the T-cell recall response to the encephalitogenic peptide MOG35–55. ITE treatment suppressed the recall proliferative response to MOG35–55 (Fig. 1C); no differences were seen upon activation with mitogenic antibodies to CD3 (Fig. S4), indicating that ITE treatment did not result in global immunosuppression. Moreover, draining lymph node cells from ITE-treated mice secreted higher amounts of TGF-β1 and IL-10 and lower amounts of IFN-γ and IL-17 upon activation with MOG35–55 (Fig. 1D).
To investigate the therapeutic potential of ITE, we used the relapsing-remitting model of EAE induced in SJL mice by immunization with PLP139–151. We initiated treatment with ITE on day 17 (daily, 200 μg/mice, intraperitoneally), during the remission that followed the first attack. Treatment with ITE reduced the disability in the relapse and the encephalitogenic response to PLP139–151 (Fig. 1 E and F). Thus, AHR activation by ITE suppresses the encephalitogenic T-cell response and EAE in both preventive and therapeutic experimental paradigms.
AHR Activation by the Nontoxic Endogenous Ligand ITE Expands the FoxP3+ Treg Compartment.
We (3) and others (4, 5, 7, 10) have shown that AHR activation in vivo can expand the FoxP3+ Treg compartment; thus, we investigated the effect of ITE on FoxP3+ Treg. We found that ITE administration (200 μg/mice, intraperitoneally) to Foxp3gfp mice starting on the day of EAE induction increased the frequency of CD4+ FoxP3:GFP+ Treg, mainly CD4+ CD25+ FoxP3:GFP+ Treg (Fig. 2A). Similar results were observed in SJL mice treated with ITE from day 17 after EAE induction (Fig. S5). Hence, ITE expands the FoxP3+ Treg compartment.
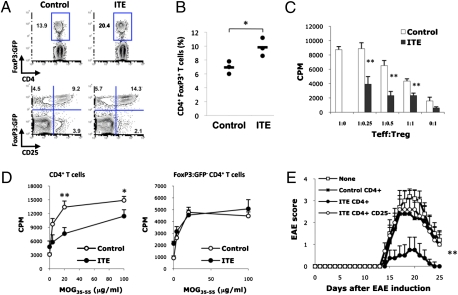
Fig. 2.
AHR activation by the nontoxic endogenous ligand ITE expands the FoxP3+ Treg compartment. (A) Frequency of CD4+ Foxp3:GFP+ Treg in splenocytes from ITE- or control-treated mice, 21 d after EAE induction. (B) CD4+ Foxp3:GFP– T cells from naive Foxp3gfp were transferred into RAG-1 deficient hosts, the recipients were immunized with MOG35–55 in IFA, treated daily with ITE for 1 wk, and the frequency of CD4+ FoxP3:GFP+ Treg was analyzed in the spleen 3 wk after immunization. *P < 0.05 compared with mice transferred with WT cells. (C) Suppressive activity of CD4+ Foxp3:GFP− Treg from ITE- or control-treated Foxp3gfp mice coincubated with naive 2D2 Foxp3:GFP− T cells activated with MOG35–55 and irradiated APC. **P < 0.001 compared with cells taken from control-treated mice. (D) Recall response to MOG35–55 of CD4+ T cells and CD4+ Foxp3:GFP− T cells lymph node cells from ITE- or control-treated Foxp3gfp mice 10 d after immunization with MOG35–55 in CFA. Cell proliferation is indicated as cpm ± SD in triplicate wells. *P < 0.05 and **P < 0.001 compared with cells taken from control-treated mice. (E) CD4+ or CD4+CD25− T cells (5 × 106) were purified from ITE- or control-treated mice 10 d after immunization with MOG35–55 in CFA and transferred into naive mice. After 1 d, EAE was induced in the recipients with MOG35–55 in CFA. The course of EAE in these mice is shown as the mean EAE score ± SEM. **P < 0.001 compared with mice transferred with CD4+ T cells from control-treated mice or CD4+ CD25− T cells from ITE-treated cells. Representative data of one of at least three experiments that produced similar results.
To further investigate the role of AHR activation by ITE on the generation of Foxp3+ iTreg in vivo, we transferred CD4+Foxp3:GFP− T cells from FoxP3gfp mice into RAG-1-deficient mice. The reconstituted mice were immunized with MOG35–55 in IFA (14) and treated daily with 200 μg per mouse ITE for 1 wk. Three weeks later the frequency of FoxP3:GFP+ Treg was analyzed by FACS. ITE treatment led to a significant increase in the conversion of CD4+Foxp3:GFP− donor T cells into CD4+Foxp3:GFP+ iTreg (Fig. 2B). Thus, the expansion of the FoxP3+ Treg compartment triggered by AHR activation with ITE is caused, at least in part, by the induction of CD4+Foxp3 + iTreg.
We then analyzed the effect of ITE on the induction of MOG35–55-specific FoxP3+ Treg. CD4+ FoxP3:GFP+ Treg from ITE or control treated mice were studied for their ability to suppress the proliferative response of 2D2+ CD4+ CD62L+ Foxp3:GFP− T cells triggered by MOG35–55 and irradiated APCs. Transgenic 2D2+ T cells express a T-cell receptor reactive with MOG35–55 (20). We found that FoxP3:GFP+ Treg from ITE-treated mice show a significant increase in their ability to suppress the proliferation of 2D2+ CD4+ CD62L+ Foxp3:GFP− T cells (Fig. 2C), suggesting that ITE expanded the MOG35–55-specific FoxP3+ Treg compartment. Moreover, to further investigate the CD4+Foxp3+ Treg, we analyzed the recall response of Foxp3gfp mice treated with ITE or vehicle. CD4+ T cells from ITE-treated mice showed a reduction in their proliferative recall response to MOG35–55 (Fig. 2D), which was abrogated by the removal of FoxP3:GFP+ T cells (Fig. 2D), suggesting that AHR activation by ITE induces FoxP3+ Treg that suppress the encephalitogenic CD4+ T-cell response.
We performed adoptive transfer experiments to study the role of Treg on the suppression of EAE by ITE administration. The transfer of 5 × 106 CD4+ T cells from ITE-treated mice before immunization with MOG35–55 resulted in a significant suppression of EAE; CD4+ T cells from control-treated mice had no effect (Fig. 2E). The suppressive activity of CD4+ T cells from ITE-treated mice was lost when CD4+CD25+ T cells were depleted (Fig. 2E). Thus, AHR activation by ITE results in the generation of CD4+Foxp3+ Treg cells that suppress the encephalitogenic response.
AHR Activation by the Nontoxic Endogenous Ligand ITE Induces Tolerogenic Dendritic Cells.
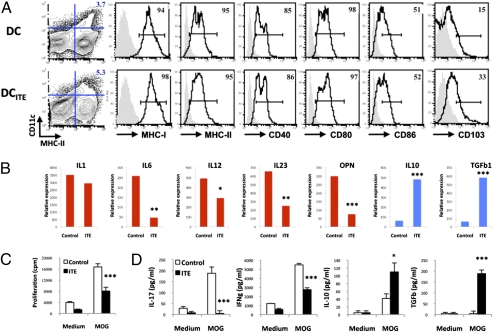
Dendritic cells (DCs) control the activation and polarization of T cells (21) and the FoxP3+ Treg compartment in vivo (22). Based on the reported expression of functional AHR by DCs (4, 23, 24), we studied the effects of ITE on DCs. To determine if DCs are affected by treatment with ITE in vivo, we immunized WT mice with MOG35–55 in complete Freund's adjuvant (CFA), treated them daily with ITE or vehicle (administered intraperitoneally), and analyzed splenic DCs by FACS 10 d after immunization. Splenic DCs from ITE-treated mice (DCITE) showed slightly decreased CD86 expression and an increased CD103 expression; no changes in the expression of MHC-I, MHC-II, CD40, and CD80 or the size of the splenic DC compartment were observed (Fig. 3A). We did find, however, that DCITE produced lower amounts of the proinflammatory cytokines IL-1β, IL-6, IL-12, IL-23, and osteopontin, and more TGF-β1 and IL-10 (Fig. 3B). DCITE also showed a decreased ability to trigger the proliferation of naive 2D2+ T cells with MOG35–55 (Fig. 3C). In addition, naive 2D2+ T cells activated with DCITE and MOG35–55 secreted lower amounts of IL-17 and IFN-γ, and more IL-10 and TGF-β1 (Fig. 3D). Hence, treatment with the AHR ligand ITE induces tolerogenic DCs in vivo.
Fig. 3.
AHR activation by the nontoxic endogenous ligand ITE induces tolerogenic DC. (A) FACS analysis of splenic DC from ITE- (DCITE) or control- (DC) treated mice. Numbers indicate the percent of positive cells; the staining obtained with isotype control antibodies is shown in gray. (B) Quantitative PCR analysis of cytokine expression by DC or DCITE. *P < 0.05; **P < 0.01; and ***P < 0.001 compared with DC. (C and D) Naive 2D2+ CD4+ FoxP3:GFP− T cells were stimulated with MOG35–55 and DC or DCITE and, and proliferation (C) and cytokine secretion (D) was analyzed. *P < 0.05; **P < 0.01; and ***P < 0.001 compared with T cells incubated with control DC. Representative data of one of at least three experiments that produced similar results.
FoxP3+ Treg can induce a tolerogenic phenotype in DCs (25). Thus, it is possible that AHR activation by ITE in vivo modifies the activity of DCs indirectly, as a result of the expansion of the FoxP3+ Treg compartment. To investigate if the tolerogenic phenotype of DCITE was a result of the direct effects of AHR activation by ITE on DCs, we generated bone marrow-derived DC (BM-DC) (26) and treated them with ITE 100 nM or vehicle during the last 24 h of their differentiation. Treatment with ITE did not affect the number or the phenotype of BM-DC at the end of the culture (Fig. S6A). However, purified ITE-treated CD11c+ BM-DC (BM-DCITE) showed a decreased expression of the proinflammatory cytokines IL-1β, IL-6, IL-12, IL-23, and osteopontin, and a concomitant increase in the expression of TGF-β1 and IL-10 (Fig. S6B). Moreover, BM-DCITE showed a reduced ability to activate 2D2 T cells with MOG35–55 and trigger the production of IL-17 or IFN-γ (Fig. S6 C and D). Conversely, 2D2 T cells activated with BM-DCITE and MOG35–55 produced increased levels of IL-10 and TGF-β1 (Fig. S6D). Taken together, these results demonstrate that AHR activation by ITE in DC induces tolerogenic DC.
DCITE Promote the Differentiation of FoxP3+ Treg by a Retinoic Acid-Dependent Mechanism.
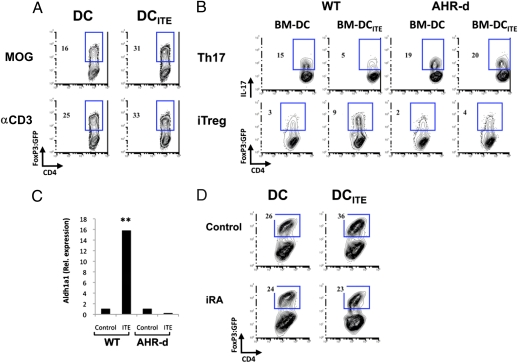
DCs drive the differentiation of effector (21) and regulatory cells (22, 27); thus, we investigated the role of AHR on the differentiation of Th17 cells and FoxP3+ iTreg by DCs. For these experiments, naive 2D2+ CD4+ CD62L+ FoxP3:GFP− T cells were activated with DCITE or control DCs in the presence of MOG35–55 or antibodies to CD3. We found that DCITE showed an increased ability to promote the differentiation of FoxP3+ iTreg upon activation with MOG35–55 or antibodies to CD3 and TGF-β1 and IL-2 (Fig. 4A). Similarly, BM-DCITE were more efficient at inducing the differentiation of FoxP3+ Treg and showed a significant decrease in their ability to promote Th17 differentiation (Fig. 4B). These effects were mediated by the activation of AHR by ITE, as they were not observed using BM-DCITE differentiated from AHR-d mice. Thus, AHR activation by ITE induces tolerogenic DCs that promote the differentiation of FoxP3+ Treg.
Fig. 4.
DCITE promote FoxP3+ Treg differentiation by an RA-dependent mechanism. (A) Naive 2D2+ CD4+ FoxP3:GFP− T cells were stimulated with DC or DCITE, MOG35–55, and TGF-β1 + IL-2, and the frequency of FoxP3:GFP+ T cells was analyzed. (B) Naive 2D2+ CD4+ FoxP3:GFP− T cells were stimulated with MOG35–55 and control (BM-DC) or ITE-treated BM-DC (BM-DCITE) derived from WT or AHR-d mice, in the presence of TGF-β1 + IL-6 or TGF-β1 + IL-2 and the frequency of IL-17+ T cells and FoxP3:GFP+ T cells was analyzed, respectively. (C) Quantitative PCR analysis of aldh1a2 expression by DC or DCITE from WT or AHR-d mice; results are presented relative to GAPDH mRNA. **P < 0.001 compared with DC from WT mice or DCITE from AHR-d mice. (D) Naive 2D2+ CD4+ FoxP3:GFP− T cells were stimulated with DC or DCITE, MOG35–55 and TGF-β1 + IL-2, with or without the specific inhibitor of RA signaling LE135 (iRA). Representative data of one of at least three experiments that produced similar results.
It has been recently reported that DCs interfere with the development of Th17 cells and promote the differentiation of FoxP3+ iTreg by means of the production of retinoic acid (RA) (11, 28, 29). The synthesis of RA is controlled by retinal dehydrogenases, which are encoded by members of the Aldh1a gene family (30). To investigate the role of RA biosynthesis in the tolerogenic phenotype of DCITE, we measured the expression of aldh1a1 and aldh1a2 in DCITE by qPCR. DCITE were found to express significantly higher levels of aldh1a1, but no changes were detected on the expression of aldh1a2 (Fig. 5C). Similar results were found when the expression of aldh1a1 and aldh1a2 was analyzed on BM-DCITE cells (Fig. S7). To investigate the significance of RA in the ability of DCITE to promote the differentiation of FoxP3+ Treg, we used the specific inhibitor of RA signaling LE135. We found that the ability of DCITE to induce the differentiation of FoxP3+ Treg was significantly reduced in the presence of LE135 (Fig. 4D); no effects were observed on the ability of control DCs to promote the differentiation of FoxP3+ Treg. Taken together, these data demonstrate that AHR activation by ITE induces anti-inflammatory DCs that promote iTreg differentiation via the secretion of RA.
Fig. 5.
Passive transfer of BM-DCITE suppresses EAE. (A) Naive mice received BM-DC and BM-DCITE (2 × 106 per mouse, three times every 4 d), derived from WT or AHR-d mice and EAE was induced. The course of EAE is shown as the mean EAE score ± SEM. **P < 0.001 compared with mice transferred with BM-DC from WT mice or BM-DCITE from AHR-d mice. (B) Recall response to MOG35–55 in splenocytes 21 d after EAE induction. Cell proliferation is indicated as cpm ± SD in triplicate wells. **P < 0.001 compared with cells from mice transferred with BM-DC from WT mice or BM-DCITE from AHR-d mice. (C) Frequency of CD4+Foxp3+ Treg in splenocytes 21 d after EAE induction, and frequency of CD4+ IL-17+ T cells and CD4+ IFN-γ+ T cells in splenocytes 21 d after EAE, following activation with MOG35–55 for 5 d. Representative data of one of at least two experiments that produced similar results.
Passive Transfer of BM-DCITE Suppresses EAE.
To investigate the relevance of the tolerogenic DCs induced by ITE treatment on the suppression of EAE, we performed adoptive transfer experiments. Control BM-DC and BM-DCITE were incubated for 1 h with MOG35–55 and transferred ip to naive mice (2 × 106 per mouse); this procedure was repeated three times, once every 4 d. Four days after the last transfer of BM-DC, EAE was induced. We found that the adoptive transfer of BM-DCITE from WT mice resulted in a significant suppression of EAE (Fig. 5A); no protective effects were observed when control BM-DC or AHR-d BM-DCITE were transferred (Fig. 5A). The suppression of EAE by WT BM-DCITE correlated with the suppression of the encephalitogenic response to MOG35–55 (Fig. 5B). BM-DC and AHR-d BM-DCITE had no effect on the recall response to MOG35–55 (Fig. 5B). Moreover, mice transferred with WT BM-DCITE had increased numbers of FoxP3+ Treg than recipients of wild type BM-DC or AHR-d BM-DCITE, and a concomitant reduction in the frequency of Th1 and Th17 cells (Fig. 5C). Thus, the DCITE induced by treatment with ITE contribute to the expansion of FoxP3+ Treg and the suppression of EAE.
Discussion
The induction of FoxP3+ Treg is viewed as a promising approach for the treatment of human autoimmune disorders (2). Several methods have been described to differentiate and expand human FoxP3+ Treg in vitro, but their ability to produce significant numbers of functional FoxP3+ Treg in a consistent manner is limited (31). Thus, strategies aimed at the induction of functional FoxP3+ Treg in vivo are more likely to be translated into clinical practice. We and others have shown that AHR activation induces functional FoxP3+ Treg that suppress the development of experimental autoimmunity and transplant rejection (3, 4, 7, 10); thus, AHR is an attractive target for the induction of functional FoxP3+ Treg. However, to date, studies on the effect of AHR ligands in models of autoimmunity have mostly focused on TCDD, a synthetic toxin. Because of its lack of toxicity (32), the endogenous AHR ligand ITE is a potential compound to induce functional FoxP3+ Treg in vivo and treat autoimmune diseases.
We found an impaired ability of AHR-d CD4+ FoxP3− T cells to differentiate into FoxP3+ iTreg both in vitro and in vivo. The mutant AHR protein in AHR-d Foxp3gfp mice displays a significant reduction in its affinity for AHR ligands (13). Thus, the reduced differentiation of AHR-d CD4+ FoxP3− T cells into FoxP3+ iTreg suggests that AHR activation in T cells by endogenous ligands plays a physiological role in the differentiation of FoxP3+ iTreg. This interpretation is in agreement with the impaired differentiation of AHR-deficient CD4+ T cells into FoxP3+ iTreg described by Kimura et al. (5). The specific AHR ligands that modulate T-cell differentiation in vitro and in vivo are, however, still largely unknown.
AHR limits the activation of Stat1 during Th17 differentiation (5), and Stat1 activation antagonizes the differentiation of FoxP3+ iTreg (17). Hence, our data suggest that by limiting the activation of Stat1, AHR interferes with the antagonistic activity of Stat1 signaling on FoxP3+ Treg differentiation. Note that Stat1 activation interferes with the differentiation of iTreg (17, 33) but not nTreg (33); indeed, several cytokines, signaling pathways, and genomic elements have differential contributions to the generation of iTreg and nTreg (34, 35). The control of Stat1 activation by AHR might preferentially favor the differentiation of FoxP3+ iTreg during an active immune response by interfering with IFN-γ signaling. Together, with the effects of AHR on foxp3 expression (3) and FoxP3+ Treg survival (4), its effects on Stat1 activation might participate in the control of immunopathology during the immune response.
AHR activation has been shown to modulate the function and maturation of DCs (4, 23, 24) and macrophages (36, 37). Indeed, AHR in macrophages limits LPS-induced inflammation (36, 37), and AHR in DCs mediates the anti-inflammatory activities of lipoxin A4 (23). We found that the AHR ligand ITE induces DCs that promote the differentiation of FoxP3+ Treg in a RA-dependent manner. Mucosal CD103+ DC promote the differentiation of FoxP3+ Treg via RA (11, 28, 29), and RA (38) and IL-10 (39) also have autocrine anti-inflammatory effects on DCs. Thus, our results support a model in which AHR activation induces tolerogenic DCs that promote the generation of FoxP3+ Treg via the production of RA and the concomitant down-regulation of proinflammatory cytokines that interfere with FoxP3+ Treg differentiation (12, 14). Moreover, it is possible that under physiological conditions, endogenous AHR ligands participate in the development of the mucosal CD103+ DC that promote the differentiation of FoxP3+ iTreg.
In conclusion, our work demonstrates that the endogenous AHR ligand ITE, given either orally or parenterally, acts on DCs and T cells to promote the induction of functional FoxP3+ Treg that suppress EAE. We recently reported that AHR activation promotes the differentiation of suppressive human regulatory T cells in vitro (40). Thus, nontoxic endogenous AHR ligands like ITE are potential new compounds for the treatment of autoimmune disorders.
Materials and Methods
Mice and Reagents.
Foxp3gfp knock-in mice have been described (12). C57BL/6-, AHR-d–, SJL-, and RAG-1–deficient mice were purchased from The Jackson Laboratories. All experiments were carried out in accordance with the guidelines of the standing committee of animals at Harvard Medical School. ITE was purchased from Sigma-Aldrich and from Tocris Bioscience.
EAE Induction.
EAE was induced by subcutaneous immunization with 100 μg of MOG35–55 peptide or 50 μg of PLP139–151 peptide (HSLGKWLGHPDKF) as described (3).
T-Cell Differentiation in Vitro.
Naive CD4+ CD62Lhigh CD44low Foxp3:GFP− T cells were stimulated for 5 to 6 d (3) using plate-bound antibody to CD3 (145-2C11, 1 μg/mL) plus soluble antibody to CD28 (PV-1, 2 μg/mL) and human TGF-β1 (3 ng/mL, unless otherwise indicated) and mouse IL-2 (50 units/mL) or mouse IL-6 (30 ng/mL). Alternatively, naive CD4 T cells were activated with BM-DC or purified DC at a 5:1 T cell-to-DC ratio, and activated with soluble antibody to CD3 (0.5 μg/mL) or MOG35–55 (20 μg/mL).
Adoptive Transfer Experiments.
RAG-1 deficient mice received 1 × 106 FACS-sorted CD4+Foxp3− T cells. One month after transfer, host mice were checked for reconstitution of CD4+ T cells, immunized with MOG35–55 in IFA, and 3 wk later, Foxp3:GFP expression was tested in splenocytes by FACS.
Real-Time PCR.
Real-time PCR was performed as described (3). All values were expressed relative to the expression of GAPDH.
Cell Proliferation and Cytokine Production.
Cells were cultured in serum-free X-VIVO 20 media (BioWhittaker) and cell proliferation and cytokine production were analyzed as described (3).
FACS.
For intracellular cytokine staining, cells were stimulated with PMA (50 ng/mL) (Sigma-Aldrich), ionomycin (1 μg/mL) (Calbiochem), and GolgiStop (BD Biosciences) for 4 h and stained, as described (3). For the analysis of Stat phosphorylation, T cells were activated with plate bound antibodies to CD3 and CD28, with or without the addition of cytokines, and 48 h after the initiation of the cultures the cells were stained with antibodies to Stat1 or Stat5, following the manufacturer's instructions (BD Biosciences).
Generation of BM-DC.
Isolated BM cells were cultured for 6 d in the presence of IL-4 (10 ng/mL) and GM-CSF (10 ng/mL). On day 6, the cells were treated with ITE or vehicle, and 24 h later, they were analyzed by FACS or purified with CD11c+ beads (Miltenyi).
Supplementary Material
Acknowledgments
We thank Deneen Kozoriz for the FACS sorting. This work was supported by Grants AI435801 and NS38037 from the National Institutes of Health (to H.L.W.), Grants 1K99AI075285 from the National Institutes of Health and RG4111A1 from the National Multiple Sclerosis Society (to F.J.Q.), and Grant MI 1221/1-1 from the Deutsche Forschungsgemeinschaft (to M.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. H.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009201107/-/DCSupplemental.
See Commentary on page 20597.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nat Rev Immunol. 2007;7:650–654. doi: 10.1038/nri2137. [DOI] [PubMed] [Google Scholar]
- 3.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 4.Hauben E, et al. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood. 2008;112:1214–1222. doi: 10.1182/blood-2007-08-109843. [DOI] [PubMed] [Google Scholar]
- 5.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, et al. Suppression of experimental autoimmune uveoretinitis by inducing differentiation of regulatory T cells via activation of aryl hydrocarbon receptor. Invest Ophthalmol Vis Sci. 2010;51:2109–2117. doi: 10.1167/iovs.09-3993. [DOI] [PubMed] [Google Scholar]
- 8.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apetoh L, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerkvliet NI, et al. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy. 2009;1:539–547. doi: 10.2217/imt.09.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 13.Okey AB, Vella LM, Harper PA. Detection and characterization of a low affinity form of cytosolic Ah receptor in livers of mice nonresponsive to induction of cytochrome P1-450 by 3-methylcholanthrene. Mol Pharmacol. 1989;35:823–830. [PubMed] [Google Scholar]
- 14.Korn T, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 17.Wei J, et al. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song J, et al. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci USA. 2002;99:14694–14699. doi: 10.1073/pnas.232562899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith AG, Francis JE, Kay SJ, Greig JB. Hepatic toxicity and uroporphyrinogen decarboxylase activity following a single dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin to mice. Biochem Pharmacol. 1981;30:2825–2830. doi: 10.1016/0006-2952(81)90421-4. [DOI] [PubMed] [Google Scholar]
- 20.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 22.Darrasse-Jèze G, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machado FS, et al. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med. 2006;12:330–334. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- 24.Platzer B, et al. Aryl hydrocarbon receptor activation inhibits in vitro differentiation of human monocytes and Langerhans dendritic cells. J Immunol. 2009;183:66–74. doi: 10.4049/jimmunol.0802997. [DOI] [PubMed] [Google Scholar]
- 25.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 26.Murugaiyan G, Agrawal R, Mishra GC, Mitra D, Saha B. Functional dichotomy in CD40 reciprocally regulates effector T cell functions. J Immunol. 2006;177:6642–6649. doi: 10.4049/jimmunol.177.10.6642. [DOI] [PubMed] [Google Scholar]
- 27.Ilarregui JM, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 28.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 29.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin Immunol. 2009;21:8–13. doi: 10.1016/j.smim.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Tran DQ, Shevach EM. Therapeutic potential of FOXP3(+) regulatory T cells and their interactions with dendritic cells. Hum Immunol. 2009;70:294–299. doi: 10.1016/j.humimm.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry EC, Bemis JC, Henry O, Kende AS, Gasiewicz TA. A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo. Arch Biochem Biophys. 2006;450:67–77. doi: 10.1016/j.abb.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Chang J-H, Kim Y-J, Han S-H, Kang C-Y. IFN-gamma-STAT1 signal regulates the differentiation of inducible Treg: potential role for ROS-mediated apoptosis. Eur J Immunol. 2009;39:1241–1251. doi: 10.1002/eji.200838913. [DOI] [PubMed] [Google Scholar]
- 34.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: More of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimura A, et al. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekine H, et al. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol Cell Biol. 2009;29:6391–6400. doi: 10.1128/MCB.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada Y, Hisamatsu T, Kamada N, Okamoto S, Hibi T. Retinoic acid contributes to the induction of IL-12-hypoproducing dendritic cells. Inflamm Bowel Dis. 2009;15:1548–1556. doi: 10.1002/ibd.20934. [DOI] [PubMed] [Google Scholar]
- 39.Kawakami Y, et al. Regulation of dendritic cell maturation and function by Bruton's tyrosine kinase via IL-10 and Stat3. Proc Natl Acad Sci USA. 2006;103:153–158. doi: 10.1073/pnas.0509784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gandhi R, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010;11:846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.