Figure 1.
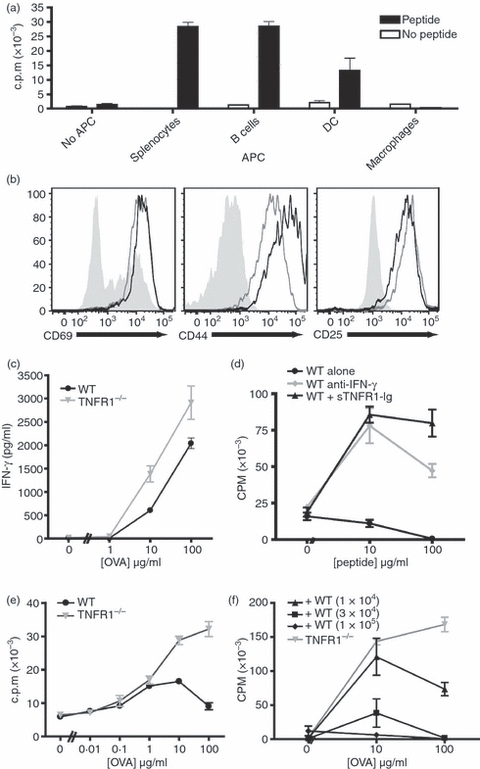
Antigen presentation by macrophages activates both T cells and macrophages, and inhibits T cell proliferation in a tumour necrosis factor-α (TNF-α) dependent manner. Ovalbumin (OVA) -specific OT-II CD4+ T cells were purified and co-cultured at a 1 : 1 ratio with a variety of syngeneic antigen-presenting cells (APCs) for 72 hr in the presence or absence of 10 μg/ml OVA peptide. The APCs tested were whole splenocytes, purified B cells, and dendritic cells (DCs) and macrophages (Mϕ) derived ex vivo from bone marrow cells. T-cell activation assessed by proliferation (a). The activation of CD4+ cells co-cultured with Mϕ (black lines), or with splenocytes (grey lines) is similar compared with naive CD4+ cells (filled grey) (b). Wild-type (WT) or tumour necrosis factor receptor 1 deficient (TNFR1−/−) Mϕ elicit similar levels of interferon-γ (IFN-γ) production by T cells 72 hr after activation (c). Mϕ-dependent inhibition of T-cell proliferation is prevented by blocking with anti-IFN-γ neutralizing monoclonal antibody (10 μg/ml) (grey circles) or sTNFR1-immunoglobulin fusion protein (10 ng/ml) (black triangles) compared with untreated control cultures (black circles). (d). OT-II T cells were co-cultured with TNFR1−/− Mϕ or WT Mϕ across a range of peptide concentrations (e). OT-II T cells were co-cultured with TNFR1−/− Mϕ (1 × 105 cells/well) and WT Mϕ in increasing numbers as indicated (f). These data are representative of three independent experiments.
