Abstract
While some probiotic strains might have adjuvant effects in the therapy for inflammatory bowel diseases (IBD), these effects remain controversial and cannot be generalized. In this study, a dltD mutant of the model probiotic Lactobacillus rhamnosus GG (LGG), having a drastic modification in its lipoteichoic acid (LTA) molecules, was analysed for its effects in an experimental colitis model. Dextran sulphate sodium (DSS) was used to induce either moderate to severe or mild chronic colitis in mice. Mice received either phosphate-buffered saline (PBS), LGG wild-type or the dltD mutant via the drinking water. Macroscopic parameters, histological abnormalities, cytokine and Toll-like receptor (TLR) expression were analysed to assess disease activity. LGG wild-type did not show efficacy in the different experimental colitis set-ups. This wild-type strain even seemed to exacerbate the severity of colitic parameters in the moderate to severe colitis model compared to untreated mice. In contrast, mice treated with the dltD mutant showed an improvement of some colitic parameters compared to LGG wild-type-treated mice in both experimental models. In addition, treatment with the dltD mutant correlated with a significant down-regulation of Toll-like receptor-2 expression and of downstream proinflammatory cytokine expression in the colitic mice. These results show that molecular cell surface characteristics of probiotics are crucial when probiotics are considered for use as supporting therapy in IBD.
Keywords: dextran sulphate sodium, inflammatory bowel disease, lipoteichoic acid, probiotic–host interaction, Toll-like receptors
Introduction
Inflammatory bowel diseases (IBD), such as Crohn's disease (CD) and ulcerative colitis (UC), are chronic illnesses that involve inflammation of the intestinal tract [1]. An increased prevalence of these diseases has been documented in developed countries. It is estimated that more than 3 million people are affected in North America and Europe [2,3]. The pathogenesis of these diseases is not fully understood, but besides genetic, environmental and immunoregulatory factors, the enteric microbiota seem to play an important role. It is thought that the inflammation results from an aberrant mucosal immune response against the indigenous microbiota in genetically susceptible hosts [4]. Additionally, it has been found that IBD is linked to an altered microbiota composition (dysbiosis) [5]. Among the mechanisms by which bacteria may promote inflammatory signalling, recent evidence suggests that microbe-associated molecular patterns (MAMPs) derived from intestinal bacteria may modulate IBD via stimulation of their respective innate immune receptors, including Toll-like receptors (TLRs) [6]. This is reflected, for example, by the dysregulation of several TLRs and susceptibility genes, such as nucleotide-binding oligomerization domain-containing 2 (NOD2), in colitis [7].
Some probiotics, which are defined as ‘live micro-organisms that when administered in adequate amounts can confer a health benefit on the host’[8], have been suggested to help in restoring the imbalances associated with IBD [9,10]. Therefore, probiotics might be useful as supporting therapeutic agents, although the results of clinical trials were not always unambiguous [10–12]. A crucial factor might be the choice of the probiotic strain. One of the best-documented and model probiotic strains is Lactobacillus rhamnosus GG (LGG) [13]. Well-substantiated health effects include prevention of acute diarrhoea in children [14], prevention of antibiotic-associated diarrhoea [15–17], prevention of atopic disease [18] and treatment of recurrent Clostridium difficile-associated colitis [19]. In IBD patients, most promising clinical effects with LGG are in prevention of pouchitis [20] and maintenance of remission in UC [21], while clinical studies with LGG in patients with CD did not result in positive outcomes [22–24].
Some molecules of LGG have been suggested to be important for the probiotic effects based on in vitro studies. For example, two secreted proteins of LGG were demonstrated to prevent cytokine-induced apoptosis in intestinal epithelial cells [25]. In addition, LGG DNA was shown recently to induce anti-inflammatory signalling via Toll-like receptor (TLR)-9 in polarized intestinal epithelial cells [26]. However, the lack of efficacy of LGG in several clinical trials with IBD patients [22–24,27] and in animal models of colitis [28,29] suggests that LGG contains factors that confound its anti-inflammatory effects in vivo.
Lipoteichoic acid (LTA) is a macroamphiphilic molecule anchored in the cytoplasmic membrane through its glycolipid moiety. It consists of a glycerol-phosphate or ribitol-phosphate chain decorated with d-alanine ester or glycosyl substitutions, and extending into the cell wall [30]. It is generally regarded as a proinflammatory bacterial molecule. LTA can be seen as the Gram-positive counterpart of Gram-negative lipopolysaccharides (LPS) [31,32], as both molecules stimulate macrophages to secrete proinflammatory cytokines in vitro, although LTA is generally less active [33]. The in vivo importance of the proinflammatory potential of LTA of gut bacteria is less clear. In healthy conditions, LTA does not cause excessive inflammation in the gut, as intestinal epithelial cells have developed special mechanisms to tolerate the continuous exposure to LTA of commensals in the gut lumen, such as down-regulation of TLR expression [34,35]. In the inflamed and more permeable gut of IBD patients LTA can, however, be hypothesized to activate macrophages and other inflammatory cells [36], although this needs to be substantiated further.
In the present work, we investigated the impact of a dedicated gene-knock-out mutation (dltD) on the anti-inflammatory efficacy of the probiotic strain LGG in a murine experimental colitis model. This LGG dltD mutant was constructed and characterized previously [37]. Its LTA molecules were shown to be completely devoid of d-alanine esters, drastically altering the LTA structure in situ on live LGG bacterial cells [37]. We induced colitis in mice by administration of dextran sulphate sodium (DSS) to focus on the involvement of epithelial barrier disruption and innate immunity.
Materials and methods
Animals
Pathogen-free female BALB/c and C57/BL6 mice, 6–8 weeks old, weighing 16–22 g, were obtained from Harlan (Zeist, the Netherlands). The mice were housed in conventional filter-top cages and had free access to commercial feed and water. All experiments were performed under the approval of the K. U. Leuven Animal Experimentation Ethics Committee (Project approval number 027/2008).
Bacterial strains, media and growth conditions
Lactobacillus rhamnosus GG (ATCC53103) (LGG) and its derivatives CMPG5540 (dltD mutant; tetracycline resistant) [37] and CMPG5340 (wild-type control strain used in the in vivo persistence analysis; erythromycin and tetracycline resistant) [38] were grown routinely at 37°C in de Man–Rogosa–Sharpe (MRS) medium (Difco; BD Biosciences, Erembodegem, Belgium) under static conditions. For solid medium, 15 g/l agar was used. If required, antibiotics were used at the following concentrations: 5 µg/ml of erythromycin and 10 µg/ml of tetracycline.
Survival in simulated gastric juice
Simulated gastric juice was prepared as reported previously [39]. The experiments were performed as described previously by Lebeer et al. [38].
Survival in the murine gastrointestinal (GIT) tract
To analyse the persistence capacity of the dltD mutant in vivo, a competition experiment was performed in 6–8-week-old female BALB/c mice, as described previously [38].
DSS-induced colitis model
Moderate to severe colitis was induced in 6–8-week-old female C57/BL6 mice by applying four cycles of 4 days 3% DSS (35–50 000 kDa; MP Biomedicals, Illkirch, France) followed by 3 days of normal drinking water [40]. Mild chronic colitis was induced by applying three cycles of 7 days 1% DSS, followed by 7 days of normal drinking water. In both models, LGG wild-type and dltD mutant were administered via the drinking water at a concentration of 108 colony-forming units (CFU) per ml throughout the experiment starting 3 days before the first cycle of DSS. Samples were taken from the drinking water throughout the experiment to confirm the concentration of viable cells. Plain phosphate-buffered saline (PBS) was used as a control. The mice given DSS were divided randomly into three treatment groups (PBS, LGG wild-type and dltD mutant) and their body weight was monitored daily. Mice were killed by cervical dislocation 29 days (3% DSS model) or 43 days (1% DSS model) after induction of colitis. The entire colon (caecum to anus) was removed and colon length was measured from the ileocaecal junction to the anus. The macroscopic scoring was based on the scoring of Mourelle et al. [41], with a maximum score of 9.
Histopathological evaluation of colitis
The colon was divided into segments representing the proximal, mid- and distal colon. From each part of the colon, a piece was taken, fixed in 6% formalin, embedded in paraffin, cut into slices and stained with haematoxylin and eosin. Stained sections were analysed blindly by a pathologist (G.D.H.) using the scoring of Kojouharoff et al. [42] with a maximum of 16.
Quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR)
For qRT-PCR, the remaining part of the colon was snap-frozen in liquid nitrogen and stored at –70°C until total RNA was extracted using the RNeasy Mini Kit (Qiagen, Gaithersburg, MD, USA). First-strand cDNA synthesis was catalysed by SuperScript II RT (Invitrogen, Carlsbad, CA, USA) using 1 µg of total RNA. The enzyme was then inactivated by incubation at 70°C for 15 min. The amount of cDNA was quantified by real-time RT-PCR using specific primers for β-actin, tumour necrosis factor (TNF), interleukin (IL)-10, IL-12p40, transforming growth factor (TGF)-‘beta’ and interferon (IFN)-γ with the ABI Prism 7700 Sequence Detection System (SDS) from Applied Biosystems (Foster City, CA, USA). The sequences of the primers and TaqMan probes for murine TNF, IL-10, IL-12p40, TGF-β, IFN-γ and β-actin have been reported previously [43]. PCR was performed as described by Maerten et al. [44] and cytokine expression levels were normalized against the housekeeping gene β-actin. Expression of TLR-1, -2, -4 and -6 was analysed using Power SYBR® Green PCR Master Mix (Applied Biosystems). Data were quantified using the ΔΔCt method relative to the housekeeping genes β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The sequences of primers for murine β-actin [43], GAPDH [45] and TLR-1, -2, -4 and -6 [46] were reported previously.
Statistics
Values are presented as mean ± standard error of the mean. Macroscopic and histological scores were analysed statistically using the Mann–Whitney U-test. Differences in parametric data were evaluated by the unpaired Student's t-test. A value of P≤ 0·05 was considered to be significant.
Results
dltD mutation does not drastically alter the survival capacity of LGG in the GIT
Changing the integrity of the bacterial cell surface can impact highly upon the persistence capacity of probiotic bacteria in the GIT [47]. To exclude the possibility that a difference in probiotic efficacy between LGG wild-type and dltD mutant is due merely to a difference in survival, the impact of a dltD mutation was first investigated after simulated gastric juice challenge in vitro and after transit through the murine GIT, as described in Materials and methods. The dltD mutant did not show a reduced survival in simulated gastric juice of pH 4 (Fig. 1a), corresponding to the pH of the murine stomach [48], or in vivo in the GIT of healthy mice (Fig. 1b). In addition, both wild-type and the mutant were shown to survive the transit through the DSS-induced inflamed murine GIT in equal numbers (Fig. 1c).
Fig. 1.
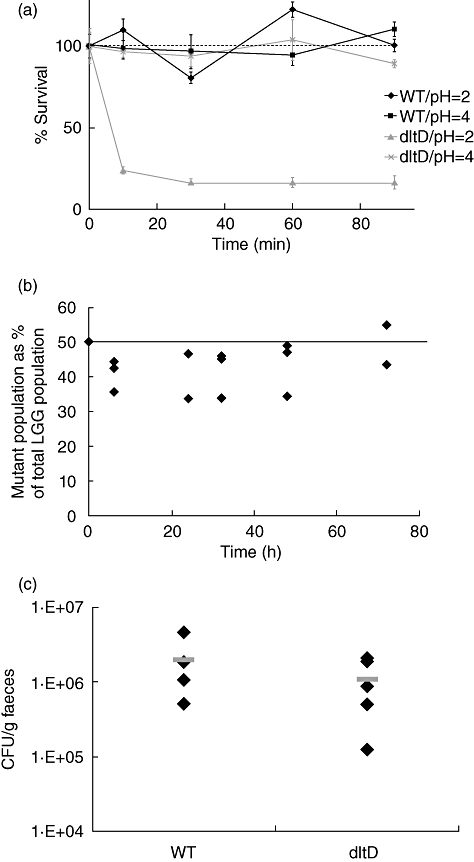
(a) Comparison of the survival of the dltD mutant (grey) and the Lactobacillus rhamnosus GG (LGG) wild-type strain (black) in simulated gastric juice at pH 2 and pH 4. The dotted line indicates that the viable cell count at start was taken as 100%. Data are the means of triplicate experiments, and error bars indicate standard error of the mean. (b) Comparison of the persistence of the dltD mutant with the wild-type control. A 1 : 1 mixture of wild-type control strain and the dltD mutant (ca. 109 colony-forming unit (CFU)/mice) was administered to three mice, indicated by the horizontal line. The percentage of mutants in the whole LGG population was determined in the fecal samples, taken at different time-points. Values for individual mice are shown. (c) Persistence of LGG wild-type and dltD mutant in 3% moderate to severe dextran sulphate sodium (DSS)-induced colitic mice. Ca. 5 × 108 CFU of each strain were administered daily to five mice. Black diamonds represent individual mice and the grey bars represent the mean value.
The dltD mutant improves colitic parameters in a moderate to severe colitis model
At the beginning, a number of pilot experiments were performed varying the concentration of DSS (from 1 to 10%), the molecular weight of DSS (35–50 kDa and 500 kDa), the murine strain (BALB/c versus C57/Bl6), the sex of the mice, the age of the mice (5–6 weeks versus 7–8 weeks) and the number of DSS administration cycles. In C57/Bl6 mice, we could establish moderate to severe colitis by cycles of 3% DSS, as specified in Materials and methods. LGG wild-type and the dltD mutant were administered via the drinking water starting 3 days before colitis induction. Daily monitoring of the body weight of the mice showed clear differences between the LGG wild-type and the mutant-treated groups (Fig. 1a). These significant differences were also observed in the macroscopic scoring after the mice were killed at day 29 post-DSS-induction; the administration of LGG wild-type seemed to aggravate the severity of colitic parameters, while the dltD mutant appeared to induce some relief (Table 1 and Fig. 2a). Mice in the PBS-treated group and in the wild-type-treated groups, in contrast with the dltD-treated group, also showed a decrease in survival, as only eight of 10 mice survived in each of these two groups (Table 1). These four mice were euthanized before the end of the experiment for ethical reasons due to severe body weight loss (unintended end-point) and were not included in the analyses of the colitic parameters. The histopathological evaluation of chosen (proximal, mid and distal) colonic segments revealed that the lesions were patchy and were found mainly in the distal part of the colon (Fig. 2b). Sections with severe mucosal damage were characterized by a loss of goblet cells, loss of crypts, epithelial cell necrosis and the local influx of inflammatory cells (mainly neutrophils) in the lamina propria and submucosa (Fig. 2b). The ‘patchiness’ of the lesions complicated the scoring and, as a consequence, no significant differences in histological scoring could be observed between the treatment groups (Table 1). Similarly, cytokine analysis by qRT-PCR did not reveal significant differences (data not shown).
Table 1.
Effect of probiotic treatment on parameters of colitis in the 3% dextran sulphate sodium (DSS) moderate to severe colitis model.
| Treatment | Macroscopic score | Histological score | Colon length (cm) | Weight gain/loss (g)* | Survival rate |
|---|---|---|---|---|---|
| PBS (control) | 5·9 ± 0·6 | 12·3 ± 1·1 | 6·2 ± 0·3 | 0·8 ± 0·5 | 8/10 |
| LGG wild-type | 6·9 ± 0·3 | 12·6 ± 0·8 | 5·4 ± 0·3 | −0·5 ± 0·7 | 8/10 |
| dltD mutant | 4·1 ± 0·6† | 12·1 ± 0·5 | 5·9 ± 0·1 | 1·4 ± 0·3† | 10/10 |
All data represent mean ± standard error of the mean [n = 8 for phosphate-buffered saline (PBS) and Lactobacillus rhamnosus GG (LGG) wild-type group, n = 10 for dltD mutant group]. Mice that did not survive were not included in the analysis of the colitic parameters.
Weight gain/loss was determined by calculating the difference in weight between day 28 (end of the experiment) and day 0 (start of the experiment). Comparison between all groups has been calculated; only significant differences are marked.
P < 0·05 between dltD-mutant and LGG wild-type-treated group.
Fig. 2.
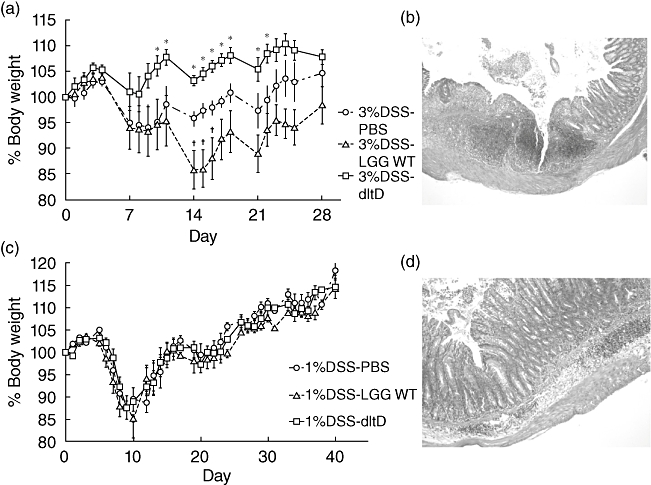
(a) Body weight curve from the 3% moderate to severe dextran sulphate sodium (DSS)-induced colitis model. Mice were divided into three groups: phosphate-buffered saline (PBS) (n = 8), Lactobacillus rhamnosus GG (LGG) wild-type (n = 8) and dltD mutant (n = 10). Body weight was monitored daily, starting from the first DSS administration. Body weight is expressed in relative values compared to the body weight at day 0. Data represent mean values ± standard error of the mean (s.e.m.). *P < 0·05 between dltD mutant and PBS-treated group; †P < 0·05 between LGG wild-type and PBS-treated group. (b) Histological photograph of an inflamed part of the colon of a PBS-treated mouse stained with haematoxylin and eosin. Mice displayed mucosal damage with a patchy pattern characterized by a loss of goblet cells and crypts, ulceration and the local influx of inflammatory cells into the lamina propria and submucosa (original magnification ×50). (c) Body weight curve from the 1% DSS-induced colitis model. Mice were divided into three groups (PBS, LGG wild-type and dltD mutant). Data represent mean values ± s.e.m. (d) Histopathological section of the colon of a mouse from the PBS-treated group receiving 1% DSS for three cycles (7 days DSS–7 days normal drinking water). Mice showed mucosal damage, restricted mainly to loss of goblet cells and local influx of inflammatory cells into the lamina propria and submucosa (original magnification ×100).
The dltD mutant also improves colitic parameters in a mild chronic colitis model
As described above, in the 3% DSS-induced model the epithelial layer was severely damaged with patchy lesions (Fig. 2b), and the administration of LGG wild-type was shown to be detrimental, in contrast to administration of the dltD mutant (Fig. 2a and Table 1). Because Yan et al. [25] reported that the intestinal epithelial cells are an important target for certain probiotic actions of LGG, we investigated subsequently whether the detrimental effect of LGG wild-type and the enhanced efficacy of the dltD mutant correlated with the integrity of the intestinal barrier. Hereto, C57/BL6 mice received 1% DSS for three cycles of 7 days DSS–7 days normal drinking water. LGG wild-type and dltD mutant were then given in the drinking water starting 3 days before colitis induction. In this model, there was no significant difference in body weight between the PBS-, wild-type- and dltD-treated groups (Fig. 2c). However, the dltD-mutant treated group showed a significantly attenuated colonic inflammation based on the macroscopic score (Table 2). In this milder model, epithelial damage was much less pronounced than in the 3% DSS-induced model, although colitis lesions were still clearly visible (Fig. 2d). Interestingly, the LGG wild-type also showed a trend of ameliorating the severity of the colitic parameters in this mild chronic model, although no significant difference could be observed compared to the PBS-treated group. qRT-PCR results revealed that the administration of the dltD mutant reduced mucosal IL-12p40 mRNA expression compared to the PBS-treated (P = 0·0170) and LGG wild-type-treated groups (P = 0·0363) (Fig. 3a). IFN-γ expression was also reduced in the dltD-treated group and this was significant compared to the PBS-treated group (P = 0·0276) (Fig. 3b). As these differences in cytokine expression might be downstream effects of a different TLR expression, we subsequently determined TLR-1, TLR-2, TLR-4 and TLR-6 mRNA expression in the three treatment groups. Mice treated with the dltD mutant showed a reduced expression of TLR-2 compared to PBS-treated mice (P = 0·0006) (Fig. 3c). Compared to LGG wild-type-treated mice, we also observed lower expression levels of TLR-1 (P = 0·0179), TLR-2 (P = 0·0328) and TLR-4 (P = 0·0443) in the dltD treated group (Fig. 3c–e). No significant differences in cytokines TNF, IL-1β, IL-10 and TGF-β were seen (data not shown). Also no significant changes in expression of TLR-6 (Fig. 3f) were observed between the three treatment groups.
Table 2.
Effect of probiotic treatment on colitis severity in the 1% dextran sulphate sodium (DSS) mild colitis model.
| Treatment | Macroscopic score | Histological score | Colon length (cm) | Weight gain/loss (g)* | Survival rate |
|---|---|---|---|---|---|
| PBS (control) (n = 10) | 7·5 ± 0·4 | 11·5 ± 0·5 | 7·1 ± 0·2 | 3·2 ± 0·4 | 10/10 |
| LGG wild-type (n = 10) | 6·7 ± 0·4 | 11·1 ± 0·5 | 6·8 ± 0·1 | 2·8 ± 0·4 | 10/10 |
| dltD mutant (n = 5) | 5·6 ± 0·6† | 12·6 ± 0·9 | 7·0 ± 0·1 | 2·8 ± 0·5 | 5/5 |
All data represent mean ± standard error of the mean.
Weight gain/loss was determined by calculating the difference in weight between day 28 (end of the experiment) and day 0 (start of the experiment). Comparison between all groups has been calculated; only significant differences are marked.
P < 0·05 between dltD mutant and phosphate-buffered saline (PBS)-treated group. LGG: Lactobacillus rhamnosus GG.
Fig. 3.
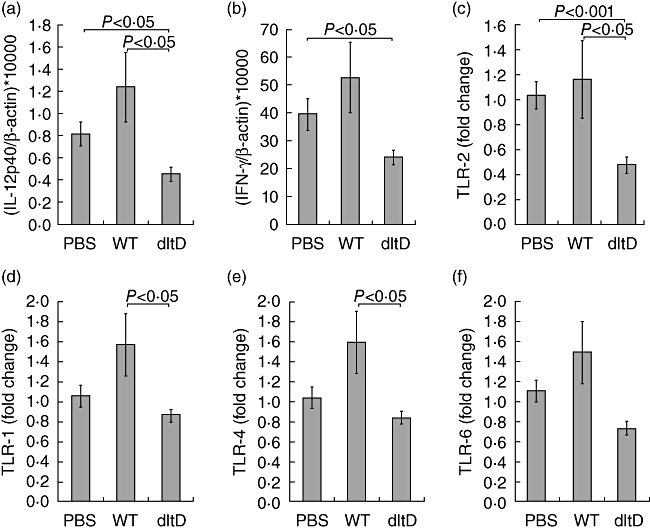
Cytokine quantification in the colon of dextran sulphate sodium (DSS)-induced colitis mice (1% DSS model). Mice were given phosphate-buffered saline (PBS), Lactobacillus rhamnosus GG (LGG) wild-type or dltD mutant and killed at day 43 after induction of colitis. Interleukin (IL)-12p40 (a), interferon (IFN)-γ (b), Toll-like receptor (TLR)-2 (c), TLR-1 (d), TLR-4 (e) and TLR-6 (f) were quantified by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) as described in Materials and methods. The expression was normalized against the housekeeping gene β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). IL-12p40 and IFN-γ were analysed with TaqMan probes and TLR-expression was measured by SYBR Green (2-ΔΔCt method). Data represent mean values ± standard error of the mean.
Discussion
Although LGG has potential as an adjuvant in the treatment of IBD, the studies are not always univocal [20–24]. For an optimized and more focused application of LGG – and other probiotics – in IBD, more knowledge about the molecular mechanisms of action is needed. Bacterial cell surface molecules are expected to be key players in determining strain-specific probiotic–host interactions [49]. As LTA is presumed to be a major proinflammatory molecule in Gram-positive bacteria [31], we studied the importance of LGG's LTA structure for its probiotic effects in a murine colitis model by using a mutant that shows a drastic LTA modification. Instead of complete removal of LTA a modification of LTA was introduced, as LTA is an essential part of the cell wall and mutants lacking LTA are not viable [50]. This LGG dltD mutant contains LTA molecules that are completely devoid of D-Ala ester substituents, resulting in an altered cell surface charge and altered cell morphology (for details see [37]).
In this work, the performance of LGG wild-type and dltD mutant was compared in two experimental set-ups of DSS-induced colitis after confirming that the mutation had no significant effect on survival. In both set-ups, the dltD mutant performed better than LGG wild-type, i.e. this mutant appeared to relieve the severity of colitic parameters. LGG wild-type exacerbated the colitic parameters in the moderate to severe model, but this detrimental effect was not seen in the mild chronic model. We hypothesize that these results could be due to severe disruption of the epithelial barrier by DSS in the moderate to severe colitis model, which was much less pronounced in the mild chronic model. One of the suggested results of this disruption is the increased passage of bacteria (including probiotic LGG) across the epithelial barrier, and subsequent increased internalization and processing by macrophages and dendritic cells in the lamina propria [51]. LTA and other proinflammatory bacterial cell wall components will then become increasingly able to induce a proinflammatory response in these cells.
Dysregulation of TLR expression in IBD could contribute to the proinflammatory response [51]. In the present work, we observed that application of the dltD mutant of LGG correlated with a significant down-regulation of TLR-2 expression in the mild chronic 1% DSS-induced colitis model compared to the PBS-treated group. This specific down-regulation of TLR-2 by treatment with the dltD mutant could explain the lower expression of the proinflammatory cytokines IL-12 and IFN-γ (as reviewed in [52]). The lower expression of IL-12 suggests that the dltD mutant induces fewer proinflammatory cytokines in macrophages and dendritic cells, as IL-12 is a proinflammatory cytokine that is produced mainly by these cell types [53]. DSS-induced colitis also involves the adaptive immune system, especially in more chronic experimental set-ups [54]. IFN-γ, a proinflammatory cytokine typically expressed by T helper 1 cells and known to be up-regulated in chronic DSS-induced colitis [54,55], was also suppressed in the dltD mutant-treated group compared to the PBS-treated group. In contrast, treatment with LGG wild-type results in an up-regulation of TLR-1, -2 and -4 compared to the dltD-treated group, highlighting the impact of inactivating the dltD gene.
It is known that LTA molecules of certain bacteria can induce proinflammatory signalling in macrophages by interaction with TLR-2 [56]. The exact role of d-alanylation in interaction of LTA with specific TLRs (TLR-2, TLR-6) and co-receptors (CD14, CD36) is not yet well established. Based on the crystal structure of TLR-2, the two acyl chains of LTA are suggested to interact with the lipid binding pocket of TLR-2, while the hydrophilic glycerophosphate chain is thought to be exposed to solvent or to interact with TLR-6 or another co-receptor of TLR-2 [57–59]. However, as LTA is a major cell wall compound of lactobacilli, changing the structure of LTA by removing d-alanine residues might as well effect the interactions with other surface molecules and therefore cause pleiotropic effects that can impact indirectly on the anti-inflammatory capacity of the lactobacilli. Nevertheless, our results with the dltD mutant compared to the wild-type probiotic strain are in line with those of the study by Grangette et al. [36], where a dltB mutant of L. plantarum NCIMB8826 also showed, compared to the wild-type strain, an enhanced anti-inflammatory capacity in vitro in monocytes and in a trinitrobenzene sulphonic acid (TNBS) colitis model [60]. Although both experimental set-ups (probiotic strains and colitis models) differ significantly, the study by Grangette et al. [36] and this study both suggest a key role for LTA modification in pro-/anti-inflammatory effects of probiotic lactobacilli.
Finally, the data from our experiments with LGG in the DSS-induced murine colitis model cannot be translated easily to the clinical setting, as introducing bacterial mutants in humans is not straightforward. However, it is interesting to mention that we also performed a pilot study with LGG in patients with active pouchitis (unpublished). Two patients with acute pouchitis received daily 1011 CFU/ml of LGG (Valio, Helsinki, Finland) in capsules for 4 weeks in a randomized cross-over trial (4 weeks probiotics, 4 weeks placebo). In one of the patients, the symptoms of active pouchitis seemed to be exacerbated by the treatment. This study was discontinued and we decided to focus upon animal models, such as presented in this report, to understand more clearly the interaction of LGG with the intestinal mucosa. The data from our experiments, together with reports from other research groups on animal models [28,29] and Crohn's disease patients [61], underline that caution should be taken when applying the wild-type strain of the well-known probiotic LGG in patients with active IBD. This seems to be especially important when the intestinal epithelial barrier function is impaired, as LGG could then show increased proinflammatory activation of macrophages and fewer modulatory signalling effects on intestinal epithelial cells, such as by proteins p40 and p75 [25] and DNA [26].
In conclusion, the difference in therapeutic effect between LGG wild-type and dltD mutant in vivo suggests a role for the cell surface of the wild-type LGG strain in determining its therapeutic efficacy. Interestingly, these results with the LGG dltD mutant show the potential of modifying the cell surface of probiotic strains for better treatment of IBD with probiotics. Combining these modified probiotic strains with the concept of ‘designer probiotics’[62] seems to be appealing for the future. One example of such a ‘designed’ strain is the IL-10-secreting Lactococcus lactis strain that shows potential in treatment of IBD [63,64]. Further in vitro studies are required to reveal the molecular mechanisms underlying the beneficial effects of this altered cell surface.
Acknowledgments
I.C. holds a PhD grant of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT–Vlaanderen). D.B. holds a senior researcher grant of FWO–Vlaanderen. Additionally, this work was supported partially by the FWO–Vlaanderen through project G.0236·07. We thank K. Geboes for helpful discussions regarding the set-up of the animal experiments. The authors also gratefully acknowledge L. Ophalvens for excellent technical assistance. We thank the anonymous reviewers for their helpful comments and suggestions.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–9. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 4.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 5.Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. 2009;22:292–301. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erridge C, Duncan SH, Bereswill S, Heimesaat MM. The induction of colitis and ileitis in mice is associated with marked increases in intestinal concentrations of stimulants of TLRs 2, 4, and 5. PLoS One. 2010;5:e9125. doi: 10.1371/journal.pone.0009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin A, Shibolet O. Toll-like receptors in inflammatory bowel disease-stepping into uncharted territory. World J Gastroenterol. 2008;14:5149–53. doi: 10.3748/wjg.14.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Food and Agriculture Organization/World Health Organization (FAO/WHO) Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Food and Agriculture Organization of the United Nations and World Health Organization expert consultation report. Rome, Italy: FAO/WHO; 2001. [Google Scholar]
- 9.Sheil B, Shanahan F, O’Mahony L. Probiotic effects on inflammatory bowel disease. J Nutr. 2007;137:819S–24S. doi: 10.1093/jn/137.3.819S. [DOI] [PubMed] [Google Scholar]
- 10.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–33. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Isaacs K, Herfarth H. Role of probiotic therapy in IBD. Inflamm Bowel Dis. 2008;14:1597–605. doi: 10.1002/ibd.20465. [DOI] [PubMed] [Google Scholar]
- 12.Hormannsperger G, Haller D. Molecular crosstalk of probiotic bacteria with the intestinal immune system: clinical relevance in the context of inflammatory bowel disease. Int J Med Microbiol. 2010;300:63–73. doi: 10.1016/j.ijmm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Doron S, Snydman DR, Gorbach SL. Lactobacillus GG: bacteriology and clinical applications. Gastroenterol Clin North Am. 2005;34:483–98. doi: 10.1016/j.gtc.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Allen SJ, Okoko B, Martinez E, Gregorio G, Dans LF. Probiotics for treating infectious diarrhoea. Cochrane Database Syst Rev. 2004;2:CD003048. doi: 10.1002/14651858.CD003048.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64. doi: 10.1542/peds.104.5.e64. [DOI] [PubMed] [Google Scholar]
- 16.Armuzzi A, Cremonini F, Bartolozzi F, et al. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163–9. doi: 10.1046/j.1365-2036.2001.00923.x. [DOI] [PubMed] [Google Scholar]
- 17.Cremonini F, Di Caro S, Covino M, et al. Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744–9. doi: 10.1111/j.1572-0241.2002.07063.x. [DOI] [PubMed] [Google Scholar]
- 18.Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361:1869–71. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 19.Gorbach SL, Chang TW, Goldin B. Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. Lancet. 1987;2:1519. doi: 10.1016/s0140-6736(87)92646-8. [DOI] [PubMed] [Google Scholar]
- 20.Gosselink MP, Schouten WR, van Lieshout LM, Hop WC, Laman JD, Ruseler-van Embden JG. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis Colon Rectum. 2004;47:876–84. doi: 10.1007/s10350-004-0525-z. [DOI] [PubMed] [Google Scholar]
- 21.Zocco MA, dal Verme LZ, Cremonini F, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567–74. doi: 10.1111/j.1365-2036.2006.02927.x. [DOI] [PubMed] [Google Scholar]
- 22.Prantera C, Scribano ML, Falasco G, Andreoli A, Luzi C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn's disease: a randomised controlled trial with Lactobacillus GG. Gut. 2002;51:405–9. doi: 10.1136/gut.51.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schultz M, Timmer A, Herfarth HH, Sartor RB, Vanderhoof JA, Rath HC. Lactobacillus GG in inducing and maintaining remission of Crohn's disease. BMC Gastroenterol. 2004;4:5. doi: 10.1186/1471-230X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bousvaros A, Guandalini S, Baldassano RN, et al. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn's disease. Inflamm Bowel Dis. 2005;11:833–9. doi: 10.1097/01.mib.0000175905.00212.2c. [DOI] [PubMed] [Google Scholar]
- 25.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–75. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghadimi D, Vrese M, Heller KJ, Schrezenmeir J. Effect of natural commensal-origin DNA on toll-like receptor 9 (TLR9) signaling cascade, chemokine IL-8 expression, and barrier integritiy of polarized intestinal epithelial cells. Inflamm Bowel Dis. 2010;16:410–27. doi: 10.1002/ibd.21057. [DOI] [PubMed] [Google Scholar]
- 27.Kuisma J, Mentula S, Jarvinen H, Kahri A, Saxelin M, Farkkila M. Effect of Lactobacillus rhamnosus GG on ileal pouch inflammation and microbial flora. Aliment Pharmacol Ther. 2003;17:509–15. doi: 10.1046/j.1365-2036.2003.01465.x. [DOI] [PubMed] [Google Scholar]
- 28.Geier MS, Butler RN, Giffard PM, Howarth GS. Lactobacillus fermentum BR11, a potential new probiotic, alleviates symptoms of colitis induced by dextran sulfate sodium (DSS) in rats. Int J Food Microbiol. 2007;114:267–74. doi: 10.1016/j.ijfoodmicro.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Mileti E, Matteoli G, Iliev ID, Rescigno M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS One. 2009;4:e7056. doi: 10.1371/journal.pone.0007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev. 2003;67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis. 2002;2:171–9. doi: 10.1016/s1473-3099(02)00226-8. [DOI] [PubMed] [Google Scholar]
- 32.von Aulock S, Morath S, Hareng L, et al. Lipoteichoic acid from Staphylococcus aureus is a potent stimulus for neutrophil recruitment. Immunobiology. 2003;208:413–22. doi: 10.1078/0171-2985-00285. [DOI] [PubMed] [Google Scholar]
- 33.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Melmed G, Thomas LS, Lee N, et al. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406–15. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 35.Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054–70. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Grangette C, Nutten S, Palumbo E, et al. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci USA. 2005;102:10321–6. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perea Velez M, Verhoeven TL, Draing C, et al. Functional analysis of d-alanylation of lipoteichoic acid in the probiotic strain Lactobacillus rhamnosus GG. Appl Environ Microbiol. 2007;73:3595–604. doi: 10.1128/AEM.02083-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lebeer S, Claes IJ, Verhoeven TL, et al. Impact of luxS and suppressor mutations on the gastrointestinal transit of Lactobacillus rhamnosus GG. Appl Environ Microbiol. 2008;74:4711–18. doi: 10.1128/AEM.00133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corcoran BM, Stanton C, Fitzgerald GF, Ross RP. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl Environ Microbiol. 2005;71:3060–7. doi: 10.1128/AEM.71.6.3060-3067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 41.Mourelle M, Guarner F, Malagelada JR. Polyunsaturated phosphatidylcholine prevents stricture formation in a rat model of colitis. Gastroenterology. 1996;110:1093–7. doi: 10.1053/gast.1996.v110.pm8612998. [DOI] [PubMed] [Google Scholar]
- 42.Kojouharoff G, Hans W, Obermeier F, et al. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107:353–8. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- 44.Maerten P, Kwon BS, Shen C, et al. Involvement of 4-1BB (CD137)-4-1BBligand interaction in the modulation of CD4 T cell-mediated inflammatory colitis. Clin Exp Immunol. 2006;143:228–36. doi: 10.1111/j.1365-2249.2005.02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen JJ, Holt L, Sartor RB. Gene expression patterns in experimental colitis in IL-10-deficient mice. Inflamm Bowel Dis. 2009;15:890–9. doi: 10.1002/ibd.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–53. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lebeer S, Vanderleyden J, De Keersmaecker SC. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev. 2008;72:728–64. doi: 10.1128/MMBR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McConnell EL, Basit AW, Murdan S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J Pharm Pharmacol. 2008;60:63–70. doi: 10.1211/jpp.60.1.0008. [DOI] [PubMed] [Google Scholar]
- 49.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8:171–84. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 50.Rahman O, Dover LG, Sutcliffe IC. Lipoteichoic acid biosynthesis: two steps forwards, one step sideways? Trends Microbiol. 2009;17:219–25. doi: 10.1016/j.tim.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–44. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 52.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 53.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–96. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- 54.Dieleman LA, Palmen MJ, Akol H, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–91. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ito R, Shin-Ya M, Kishida T, et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol. 2006;146:330–8. doi: 10.1111/j.1365-2249.2006.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuguchi T, Takagi A, Matsuzaki T, et al. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol. 2003;10:259–66. doi: 10.1128/CDLI.10.2.259-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin MS, Kim SE, Heo JY, et al. Crystal structure of the TLR1–TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–82. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Nilsen NJ, Deininger S, Nonstad U, et al. Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling: role of CD14 and CD36. J Leukoc Biol. 2008;84:280–91. doi: 10.1189/jlb.0907656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang JY, Nan X, Jin MS, et al. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity. 2009;31:873–84. doi: 10.1016/j.immuni.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 60.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev. 2007;59:1073–83. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Shen J, Ran HZ, Yin MH, Zhou TX, Xiao DS. Meta-analysis: the effect and adverse events of Lactobacilli versus placebo in maintenance therapy for Crohn disease. Intern Med J. 2009;39:103–9. doi: 10.1111/j.1445-5994.2008.01791.x. [DOI] [PubMed] [Google Scholar]
- 62.Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009;136:2015–31. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steidler L, Hans W, Schotte L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–5. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 64.Braat H, Rottiers P, Hommes DW, et al. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:754–9. doi: 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]


