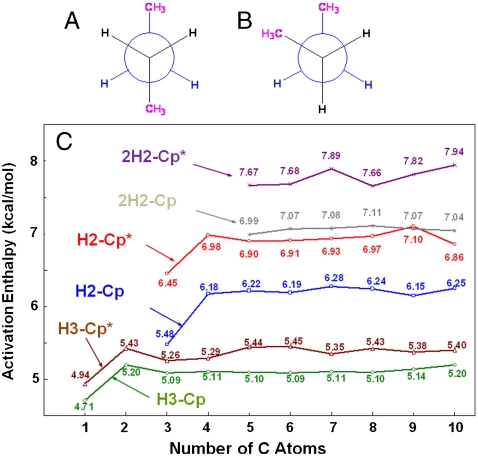
Fig. 3.
Schematic representation of (A) trans-n-butane and (B) gauche-n-butane. The different terminal C─H bonds have different activation energies because of the different orientation of the alkane chain with respect to CpRh(CO). For CpRh(CO)(alkane) the average difference between A and B is 0.71 kcal mol-1, whereas for Cp∗Rh(CO)(alkane) the average difference is 1.93 kcal mol-1. (C) Calculated C─H activation barriers for the primary (C∗H3CH2CH2─) C─H bonds activation enthalpies (in kcal mol-1) vs. number of carbon atoms for the linear alkanes (n = 1 to 10) by CpRh(CO) (H3-Cp; green curve) and by Cp∗Rh(CO) (H3-Cp*; brown curve) and the secondary C─H activation enthalpies (in kcal mol-1) vs. number of carbon atoms for the n-alkanes propane through decane by CpRh(CO) (H2-Cp; blue curve for CH3C∗H2CH2─ and 2H2-Cp; gray curve for CH3CH2C∗H2─) and by Cp*Rh(CO) (H2-Cp*; red curve for CH3C∗H2CH2─ and 2H2-Cp*; purple curve for CH3CH2C∗H2─). All data were calculated at the PBE level and offset upward by 3.43 kcal mol-1 to obtain the approximate CCSD values. The offset value of 3.43 kcal mol-1 is the activation enthalpy difference between the PBE calculation and the CCSD//PBE calculation for methane’s C─H activation by CpRh(CO).