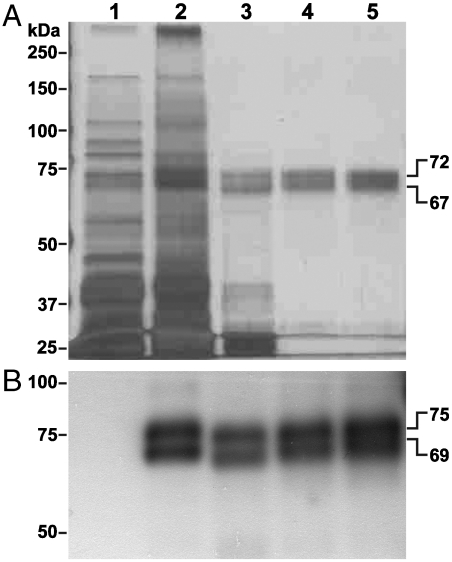
Fig. 1.
Highly purified preparation of pBChE. Water-soluble proteins were extracted from leaves of WT plants (lane 1) or transgenic plants that express pBChE (lane 2). The recombinant protein was purified from the extract by ammonium sulfate fractionation (30%–70% fraction, lane 3), and in tandem affinity chromatography steps using Con A-sepharose (lane 4) and procainamide (lane 5). Protein samples were resolved by SDS-PAGE and either (A) subjected to silver staining or (B) transferred to a PVDF membrane, immunodecorated by an anti-human BChE antibody followed by HRP-conjugated secondary antibody, and visualized by chemiluminescence. Gels were loaded based on equal BChE activity at 24 mU (A) or 2.4 mU (B). Crude extract samples contained equivalent amounts of total soluble proteins. One unit of enzyme will hydrolyze 1.0 µmol of butyrylthiocholine to thiocholine and butyrate per minute.