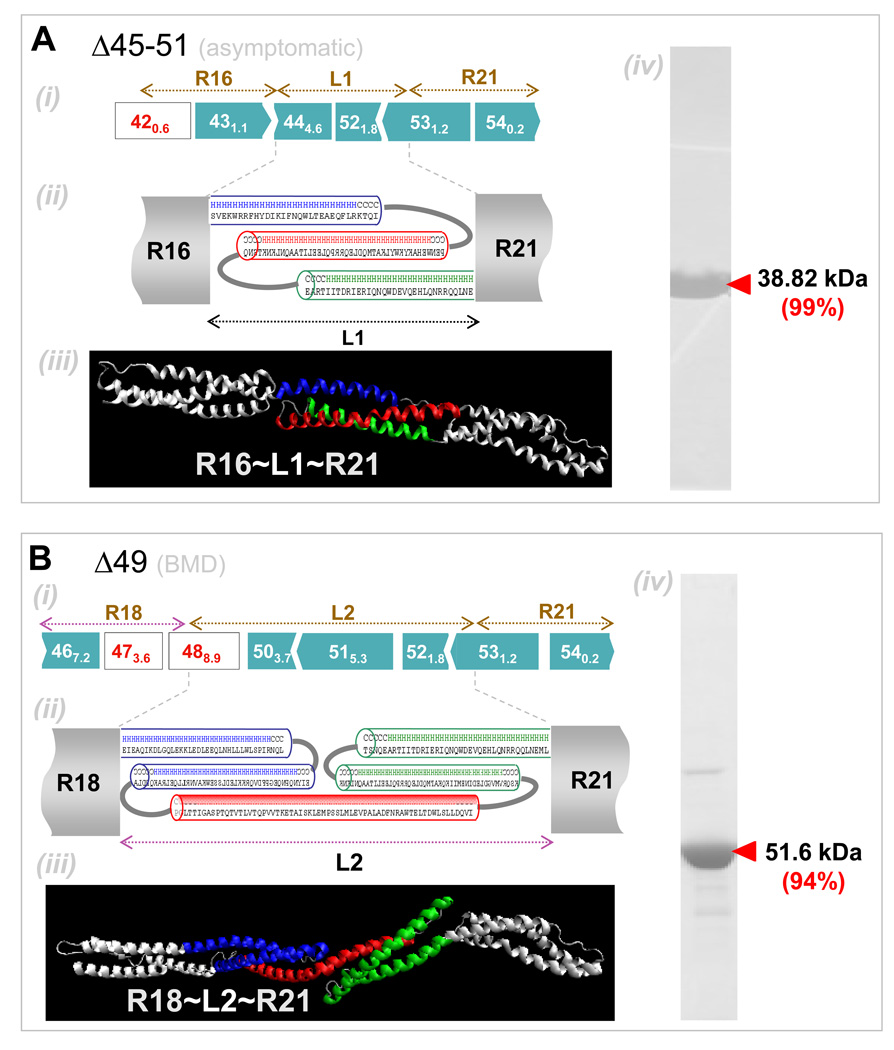
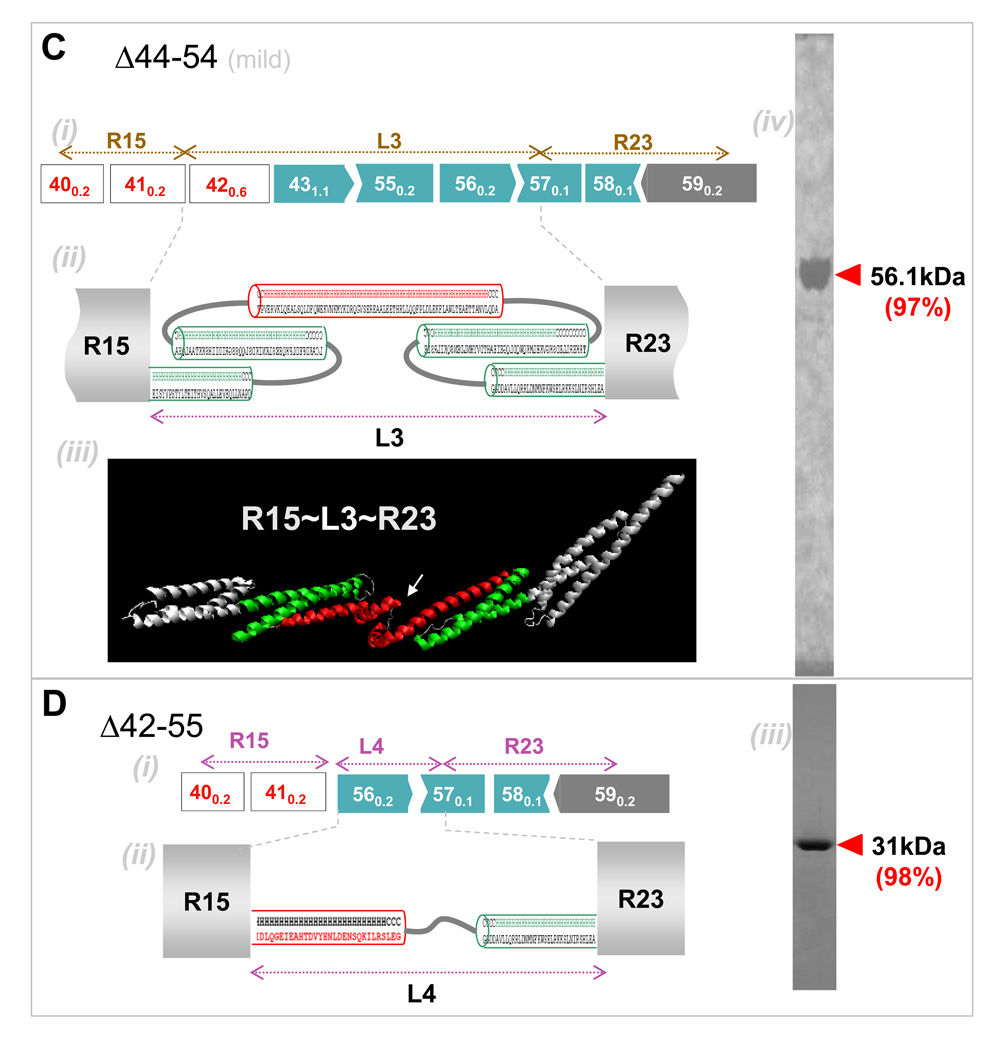
Figure 3. Secondary and tertiary structure prediction of BMD-inspired nano-dystrophin constructs.
(A) R16~L1~R21: (i) Exon map (ii) Secondary structure predictions for abnormal ‘linker’, L1, show this is mostly helical. Helical wheel predictions (not shown) indicate the presence of a hydrophobic core, necessitating packing of the helices into a spectrin-like domain. (iii) Homology model prediction for L1 with adjacent normal repeats shown in white. Color coding matches that of the structure prediction. (iv) Coomassie blue staining of purified nano-construct in SDS/PAGE, with purity in densitometry of 99%. (B) R18~L1~R21: (i) Exon map. (ii) Linker region L2 is again predicted to be mostly helical and to form two spectrin-like domains. (iii) Homology model for L3 also predicts folding into two repeat domains. (iv) Purified protein. (C) R15~L3~R23: (i) Exon map. (ii) Secondary structure predictions predict L3 to be mostly helical with two spectrin-like domains. (iii) Homology model prediction shows linker L3 might also contain another kink or hinge-like domain as indicated by a white arrow. (iv) Purified protein. (D) R15~L4~R23. (i) Exon map. (ii) The linker region was predicted to be helical, with an unstructured coil region in the middle. (iii) Purified protein.