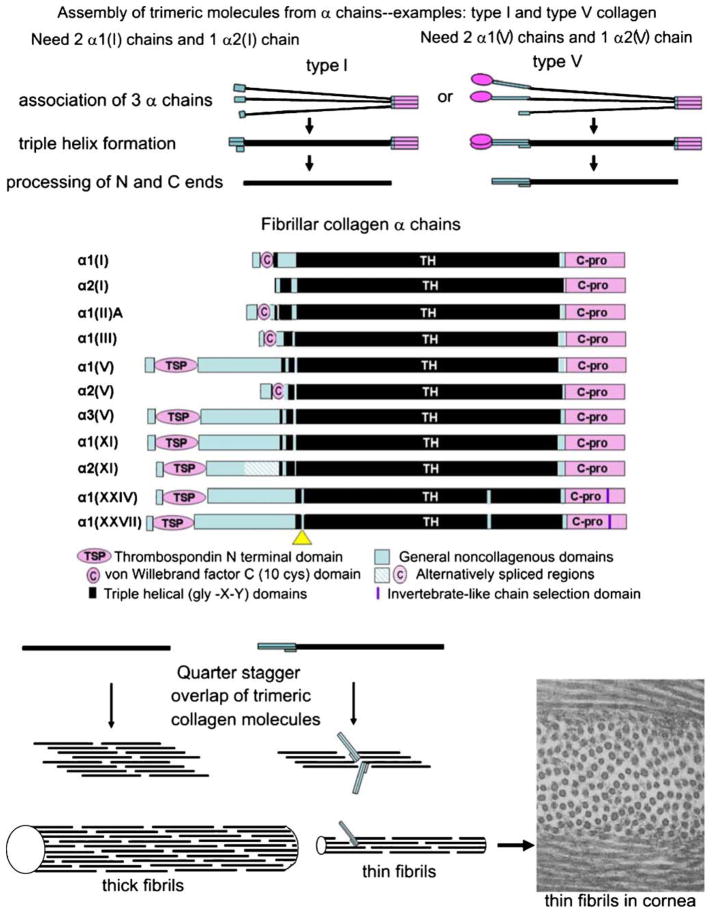
Fig. 1.
Assembly of α chains into trimeric collagen molecules and then of molecules into fibrils. Top The folding of three chains into a single molecule. Middle Fibrillar collagen α chains showing domain structures. Scale is approximate (C-pro C-propeptide domain, TH gly-X-Y repeating region). The yellow triangle is a short non-gly-X-Y domain in collagens XXIV and XXVII and has been interpreted in two ways: as a separation between the minor and major helices in collagen XXIV, and as an interruption in the major (and only) triple helix in collagen XXVII. Bottom Perfect quarter stagger overlaps result when no bulky groups protrude from the fibril surface. Bottom right Electron micrograph of collagen fibrils in the rabbit cornea. The thin diameters (~25 nm) are the result of fibrils being heterotypic structures composed of ~80% type I and 20% type V collagen. ×60,000