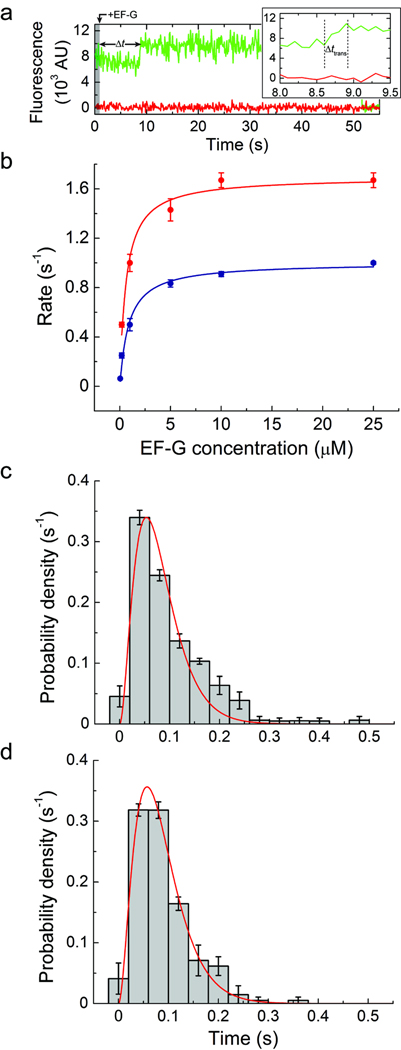
Figure 4. A step-like increase in Cy3 fluorescence accompanies peptidyl-tRNA movement to the P site.
(a) Single-molecule fluorescence trajectories (Cy3, green; Cy5, red) obtained during delivery of 10 µM unlabeled EF-G and 1 mM GTP to complexes with P-site tRNAfMet and A-site fMet-Phe-tRNAPhe(Cy3-acp3U47). (b) The rate of observing the increase in Cy3 fluorescence in complexes with either (blue) P-site tRNAfMet and A-site fMet-Phe-tRNAPhe(Cy3-acp3U47) or (red) P-site tRNAPhe and A-site NAcPhe-Lys-tRNALys(Cy3-acp3U47), across a range of EF-G concentrations (0.05–25 µM). The data were fit to the hyperbolic function Rate = Ratemax[EF − G]/(K1/2 + [EF − G]) with Ratemax = 1.0 ± 0.1 s−1 and K1/2 = 0.9 ± 0.1 µM for the case of P-site tRNAfMet, and Ratemax = 1.7 ± 0.1 s−1 and K1/2 = 0.6 ± 0.1 µM for P-site tRNAPhe. (c) The distribution of time over which the increase in Cy3 fluorescence occurs for complexes containing P-site tRNAfMet. In agreement with a previous report36, the distribution is well fit (R2 ≈ 0.95) by the distribution predicted for a model with three successive steps with equal rate constants (f(t) = (k3t2/2) exp(−kt), k = 37 ± 3 s−1). (d) The distribution obtained from complexes containing P-site tRNAPhe was fit to the same function with k = 35 ± 3 s−1 (R2 ≈ 0.94). Error bars represent the standard error.