Figure 2. Evolution of MST Devices for Non-Human Primate and Clinical Trials.
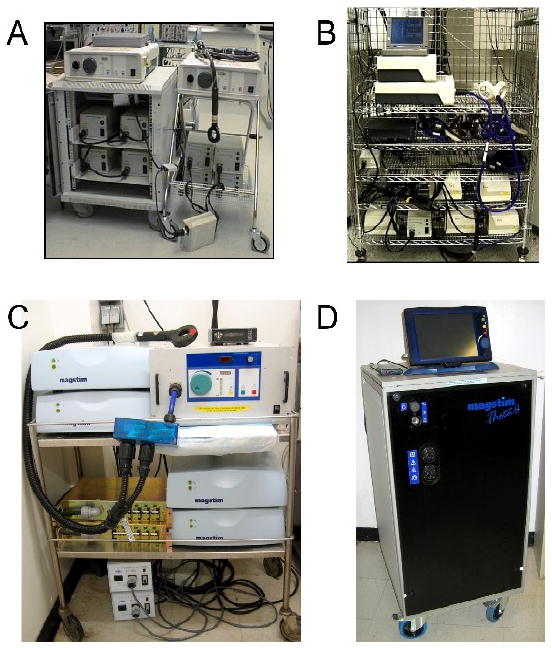
A) First generation nonhuman primate MST device, used in the first MST feasibility trials in rhesus monkeys (Lisanby, et al., 2001) and the first depressed patient (Lisanby, et al., 2001). A conventional rTMS device was expanded by adding 4 charging units, yielding a total of 8 booster modules and a maximal stimulator output of 40 Hz.
B) First generation human MST device used in the first randomized trial of MST in the treatment of depression conducted at Columbia and University of Texas Southwestern Medical Center (Lisanby, et al., 2003, White, et al., 2006). Sixteen charging units were employed, resulting in a maximal stimulator output of 50 Hz, 8 seconds, 100% intensity.
C) Second generation nonhuman primate high dose MST (HD-MST) device used in the first animal studies of HD-MST (Spellman, et al., 2008). A three-phase power supply and other revisions to circuit topology were used to achieve 100 Hz, 10 second trains at 100% intensity.
D) Second generation human HD-MST device used in the first human studies of HD-MST (Kirov, et al., 2008). The Magstim Theta is capable of 1000 pulses per train at 100 Hz and 100% intensity, as well at theta burst stimulation. Three coils can be connected simultaneously to facilitate seizure threshold titration. A touch screen monitor provides an interactive user interface. The self-contained console has a small footprint to fit easily in a typical clinical ECT suite.
