Figure 4. Translational development strategy applied to MST.
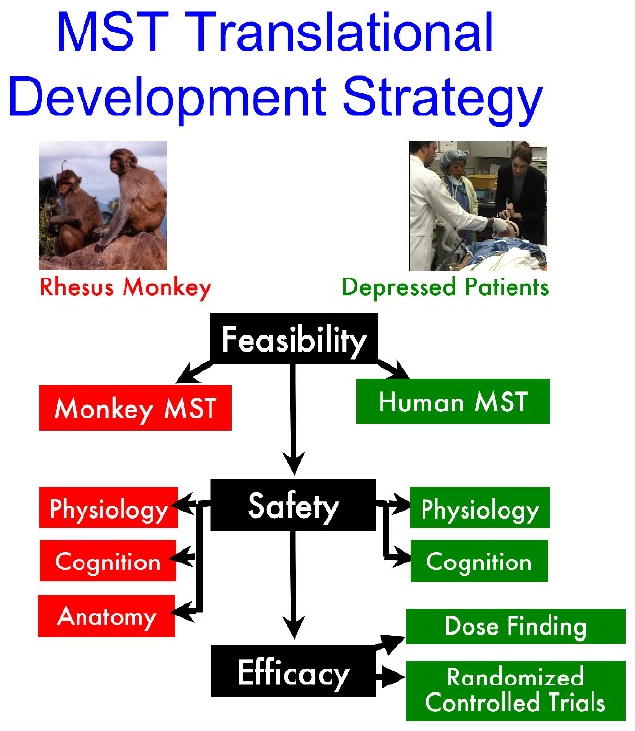
The development of MST progressed through the stages of feasibility testing, safety evaluation, and efficacy trials. At each stage, work toggled back and forth between animal testing in nonhuman primates and clinical trials. Device design was iteratively improved, incorporating results from each stage of development. Animal testing provided the unique opportunity to test the safety of MST using postmortem anatomical studies, while clinical trials provide the unique opportunity to assess efficacy in depressed patients.
