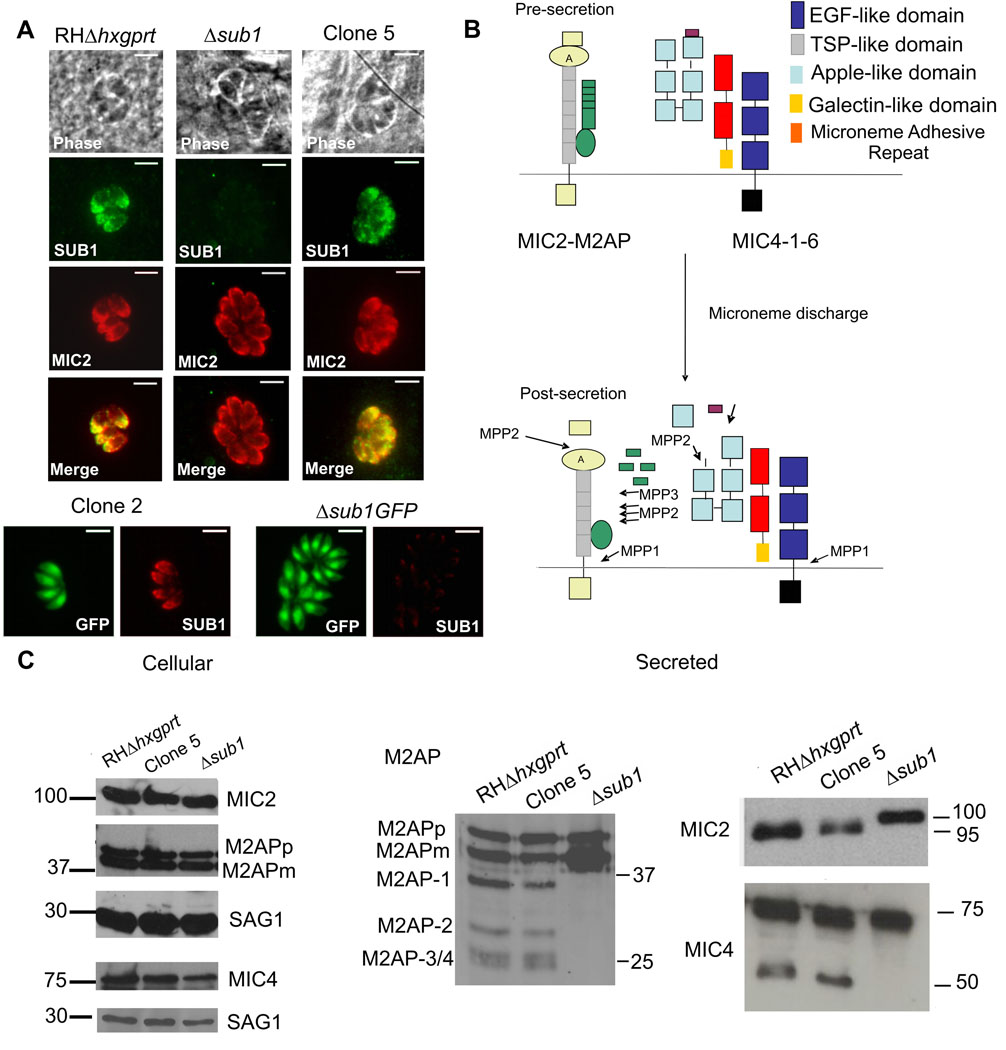
Figure 1. TgSUB1 disruption alters MIC proteolytic processing.
(A) Immunofluorescence of Δsub1 strains and sibling clones (Clone 2 for Δsub1 GFP; Clone 5 for Δsub1). Controls include the wild-type RH GFP-Δhxgprt and RHΔhxgprt strains. Siblings correspond to wild-type parasites stably transfected with the selectable marker but without TgSUB1 disruption. TgSUB1 is labeled with the TgSUB1 AE653 rabbit antiserum. MIC2 is labeled with the mouse mAb 6D10 and was used as a marker for microneme localization. Scale bar is 5 µm (B) Schematic representation of the cell surface proteolytic trimming of the micronemal adhesive complexes MIC2-M2AP and MIC1-4–6. “A” denotes the A-domain of MIC2 protein. Microneme Adhesive Repeat refers to the specialized MIC1 domain which discriminates between glycan residues (Blumenschein et al., 2007). Activity of the proteases MPP1-2–3 is represented. Because the first step in M2AP processing is not inhibited by protease inhibitors that inhibit MPP2 (ALLM, ALLN), a second protease activity termed MPP3 was proposed (Zhou et al., 2004). The MPP1 MIC cleavage sites are consistent with MPP1 being a rhomboid serine protease such as the parasite surface rhomboids TgROM 4 or TgROM5 (Brossier et al., 2005, Dowse et al., 2005, Buguliskis et al., 2010). (C) Western Blot analysis of the MIC2-4 and M2AP cellular and secreted products. Blots were probed with appropriate antibodies as described in experimental procedures. Molecular standards are in kDa. The rabbit anti-MIC4 antibody used was raised against the A1 and A2 apple domains, and recognizes the 72 and 70 kDa precursor forms and the 50 kDa secreted product but not the 15 kDa secreted product. M2APp: precursor form; M2APm: mature form.