Abstract
Objective: This study was designed to detect the changes of serum soluble Fas (sFas) levels in patients with locally advanced unresectable rectal cancer (LAURC), and to explore its prognostic value of response. Methods: Soluble samples were obtained from LAURC subjects, treated by concurrent chemoradiotherapy, before treatment and one month after treatment. Healthy donor serum samples were used as controls. sFas concentration was measured by enzyme-linked immunosorbent assay (ELISA). Results: The sFas levels before treatment and one month after treatment were both significantly higher in LAURC subjects than in healthy controls [(8.79±1.39) and (7.74±1.32) vs. (5.53±1.13) ng/L, P<0.01]. The sFas levels before treatment and one month after treatment were significantly lower in the response group (complete and partial responses) than in the non-response group (stable and progressive diseases) [(8.50±1.25) vs. (10.17±1.26) ng/L, P<0.01 and (7.50±1.24) vs. (8.90±1.13) ng/L, P<0.01, respectively]. The one-year survival rate was 54.2% and 82.6% in those with sFas levels >8.79 ng/L and <8.79 ng/L before treatment (P<0.02), respectively, 50.0% and 87.0% in those with sFas levels >7.74 ng/L and <7.74 ng/L one month after treatment (P<0.01), respectively. Conclusions: The sFas level is higher in LAURC subjects than in healthy controls. Concurrent chemoradiotherapy can reduce sFas levels in LAURC patients. The monitoring of sFas may provide prognostic information for LAURC patients.
Keywords: Soluble Fas (sFas), Rectal cancer, Concurrent chemoradiotherapy, Prognosis
1. Introduction
The morbidity and mortality of rectal cancer have been increasing worldwide in recent years (Liang et al., 2009). Surgical resection was the first choice for patients with early stage cancer; however, five-year overall survival rate was only about 60% after radical resection (Bosset et al., 2004), and the overall recurrence rate was 25%–40% (Havenga et al., 1999; Nesbakken et al., 2002). The result in locally advanced unresectable rectal cancer (LAURC) patients with chemotherapy or radiotherapy alone has been disappointing, but chemotherapy combined with radiotherapy made a remarkable improvement (Kachnic, 2007). The advantages of concurrent chemoradiotherapy might be: (1) synergistic effect between radiotherapy and chemotherapy; (2) no delayed treatment time; (3) reduction of the chance of drug-cross resistance. Research has shown that concurrent chemoradiotherapy can improve local control rates in patients with rectal cancer, thus improving the survival rate (Klautke et al., 2005); however, there is still a lack of accurate biological indicators in diagnosis, treatment, and prognosis. Fas is a key receptor triggering apoptosis in cells. Studies have reported that soluble Fas (sFas), to a certain extent, reflects the biological behavior of tumors (Liang et al., 2002), and it can be a predictive factor during radiotherapy (Zhu et al., 2006) and chemotherapy (Shimizu et al., 2005; Chaudhry et al., 2008), but there is still little evidence confirming whether concurrent chemoradiotherapy declines sFas levels and whether sFas levels have a prognostic value in LAURC. Thus, the aim of our study was to detect the changes of serum sFas level in patients with LAURC, and to explore its prognostic value of response.
2. Materials and methods
2.1. Patients’ characteristics
From Jan. 2006 to Aug. 2008, the serum samples of 47 patients with LAURC and 31 healthy donors were collected from the Department of Oncology in Affiliated Hospital of Guangdong Medical College, Zhanjiang, China. Inclusion criteria: age <75 years, histologically confirmed, presented LAURC with site, clinical stages III and IV (including recurrence) who were unwilling to have an operation or for whom radical resection was too difficult (seriously infiltrating pelvic wall, main vessels, or involving S3–S5), no evidence of distant metastases, Karnofsky performance status (KPS) score >60, expected survival time ≥3 months, and routine blood examination, renal and liver functions, and electrocardiogram all normal. Clinicopathologic data are shown in Table 1.
Table 1.
Patients’ characteristics (n=47)
| Characteristic | Valuea |
| Gender | |
| Male | 27 (57.4%) |
| Female | 20 (42.6%) |
| Age (year) | 58.6 (23–66)b |
| Pathologic type | |
| Poorly differentiated adenocarcinoma | 10 (21.3%) |
| Moderately differentiated adenocarcinoma | 15 (31.9%) |
| Well differentiated adenocarcinoma | 16 (34.0%) |
| Mucinous adenocarcinoma | 6 (12.8%) |
| Clinical stage | |
| III | 29 (61.7%) |
| IV | 18 (38.3%) |
| Sphincter-preserving | |
| Yes | 16 (34.0%) |
| No (artificial anus) | 31 (66.0%) |
| Tumor recurrence type | |
| Anastomotic recurrence | 12 (25.5%) |
| Pelvic recurrence (including the pelvic lymph nodes, uterus and accessories, vagina, presacral space) | 24 (51.1%) |
| Perineal recurrence | 11 (23.4%) |
Value are expressed as number (percentage) of patients
Value is expressed as median (range)
2.2. Treatments
Radiotherapy was delivered with photons from a linear accelerator with energy >6 MV. Computed tomography-assisted three-dimensional (3D) planning of radiation therapy was employed. The planning target volume was comprised of: upper border at the L5/S1 junction, inferior border 3 cm below the primary tumor or at the inferior aspect of the obturator foramina, anterior border 1 cm anterior to the vagina wall or bladder, posterior border 1 cm behind the anterior bony sacral margin, and lateral border 1.5 cm lateral to the widest bony margin of the true pelvic side wall. Radiotherapy was delivered with three or four fields using an isocentric technique with individually collimated field portals. The total radiation dose was 66–68 Gy (Spindler et al., 2006) in 2 Gy per fraction per day, 5 d per week. The first 46 Gy was given to the planned field, and 20–22 Gy was given to the reduced field (after reviewing magnetic resonance imaging (MRI) or computed tomography (CT), if the tumor size decreased more than 50%, consideration was given to reducing radiation field).
Radiotherapy was administered with concurrent chemotherapy with FOLFOX regimen: including 5-fluorouracil (5-Fu) 400 mg/m2, iv, Day 1; 5-Fu, 3.0 g/m2, ivgtt for 46 h, Days 1–2; leucovorin (LV), 400 mg/m2, iv, Day 1; and oxalipatin, iv, 100 mg/m2, Day 1, every 3 weeks. Chemotherapy was administered on radiotherapy Days 1–2 and 22–23, for a total of two cycles and after 3–4 weeks of concurrent radiotherapy, two or three more cycles consolidated chemotherapy.
2.3. Serum sFas level detection
Serum sFas level was detected one week before starting chemoradiotherapy and one month after finishing all the treatments. The concentration of sFas was measured by enzyme-linked immunosorbent assay (ELISA; Genzyme Corporation, USA) according to the manufacturer’s instruction.
2.4. Follow-up
The patients were followed up every month for 16 months by mail, telephone, and outpatient interviews, and all patients were hospitalized and evaluated every two or three months after treatment. The total survival time was defined as the period from beginning treatment to death or the 16th month.
2.5. Evaluation criteria
The evaluation criteria were based on response evaluation criteria in solid tumor (RECIST) criteria: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). CR plus PR cases were defined as the overall response rate. SD and PD cases were summed to calculate the overall non-response rate. Toxicity assessment was according to the World Health Organization (WHO) standards.
2.6. Statistical analysis
All analyses were performed using the SPSS 18.0 software package for Macintosh. The statistical significance of differences for the mean values of sFas concentrations was determined by Student’s t-test. The one-year survival rate was analyzed with the Kaplan-Meier method. The accumulative survival rates were compared with the log-rank test. Differences with P<0.05 were considered significant.
3. Results
3.1. Therapeutic efficacy
The overall response rate was 66.0% (31/47), CR 10 cases, PR 21 cases, SD 9 cases, PD 7 cases, and the one-year survival rate was 68.1% (32/47).
3.2. Adverse events
Treatment-related adverse reactions were neutropenia, thrombocytopenia, nausea and vomiting, radiation proctitis, nerve cacesthesia, liver function lesion, and oral mucositis, most of I–III degrees, with no treatment-related deaths (Table 2).
Table 2.
Adverse effects of treatment
| Adverse event | Number of patients |
||||
| 0* | I | II | III | IV | |
| Neutropenia | 0 | 16 | 26 | 3 | 2 |
| Thrombocytopenia | 29 | 12 | 6 | 0 | 0 |
| Nausea and vomiting | 9 | 15 | 17 | 6 | 0 |
| Radiation proctitis | 22 | 10 | 8 | 5 | 2 |
| Nerve cacesthesia | 16 | 13 | 10 | 6 | 2 |
| Liver function lesion | 25 | 11 | 10 | 1 | 0 |
| Oral mucositis | 32 | 8 | 6 | 1 | 0 |
0–IV degrees according to WHO standards
3.3. sFas levels
The mean sFas levels before and one month after treatments were: (8.79±1.39) and (7.74±1.32) ng/L in the subjects with LAURC, respectively, which were higher than that in the healthy control (5.53±1.13) ng/L (P<0.01). Comparing the sFas level before treatment with that one month after treatment, the difference was also significant (P<0.01). There were 39 cases in the response group (CR+PR) and 8 cases in the non-response group (SD+PD). The comparisons of the sFas levels between two groups before treatment and one month after treatment both had significant differences (P<0.01) (Table 3).
Table 3.
Comparisons of the changes in sFas levels in subjects with LAURC
| Group | n | sFas level* (ng/L) | P |
| Pre-treatment | |||
| Control | 31 | 5.53±1.13 | <0.01 |
| LAURC | 47 | 8.79±1.39 | |
| Response (CR+PR) | 39 | 8.50±1.25 | <0.01 |
| Non-response (SD+PD) | 8 | 10.17±1.26 | |
| Post-treatment | |||
| Control | 31 | 5.53±1.13 | <0.01 |
| LAURC | 47 | 7.74±1.32 | |
| Response (CR+PR) | 39 | 7.50±1.24 | <0.01 |
| Non-response (SD+PD) | 8 | 8.90±1.13 |
Values are expressed as mean±SD
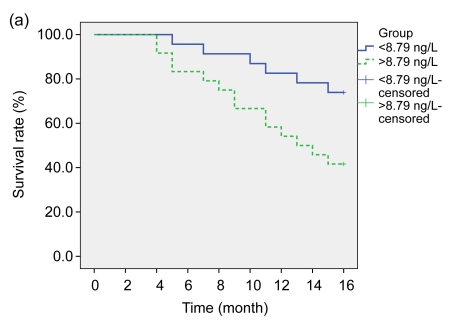
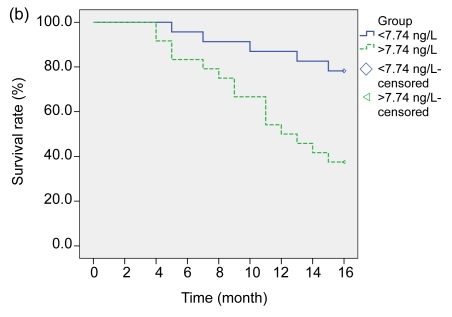
According to the changes of sFas levels before and after treatments, the subjects were further divided into four groups: <8.79 ng/L (23 cases), >8.79 ng/L (24 cases), <7.74 ng/L (23 cases), and >7.74 ng/L (24 cases), respectively, and their one-year survival rates were: 82.6% (19/23), 54.2% (13/24), 87.0% (20/23), and 50.0% (12/24) (Fig. 1).
Fig. 1.
Overall survival curves of the LAURC subjects
(a) sFas levels <8.79 ng/L and >8.79 ng/L before treatment (log-rank test: P<0.02); (b) sFas levels <7.74 ng/L and >7.74 ng/L one month after treatment (log-rank test: P<0.01)
4. Discussion
The Fas/Fas ligand (Fas/FasL) system plays an important role in regulating apoptosis. FasL (Apo-1/CD95 ligand) is a type II membrane protein having homology with tumor necrosis factor receptor (TNFR) family (Suda et al., 1993; Boroumand-Noughabi et al., 2010), which triggers target cells into apoptosis in several hours by combining with Fas or Fas monoclonal antibody inducing Fas trimerization or oligomerization (Griffith et al., 1995). Fas (Apo-1/CD95) is a type I cellular membrane protein, which belongs to the tumor necrosis factor receptor superfamily/the nerve growth factor receptor (Pan et al., 2007). Fas has two forms, a transmembrane form and a soluble form. Serum sFas is generated from cell surface that lacks 21 amino acid residues containing the transmembrane domain by alternative splicing (Petak et al., 2000). sFas inhibits the cell surface signal transduction by competitively binding and neutralizing FasL (Suda et al., 1993; Cascino et al., 1995; Natoli et al., 1995), which may correlate with cancer escaping immune surveillance and progression. Increased serum sFas level has been observed in patients with hepatocellular (Jodo et al., 1998), breast (Bewick et al., 2001), small cell lung cancer (SCLC) (Shimizu et al., 2005), esophageal (Gratas et al., 1998) and epithelial ovarian cancers (Chaudhry et al., 2008).
Radiotherapy plays an important role in the process of tumor apoptosis. Fas is the key element of radiation inducing apoptosis. Kimura and Gelmann (2000) have reported that proper γ-irradiation up-regulated cell’s surface Fas antibody and enhanced Fas receptor’s sensitivity, promoting tumor cell into apoptosis. Zhu et al. (2006) used immunohistochemistry and TdT-mediated dUTP nick end labeling (TUNEL) techniques to detect the changes of Fas, p53, Bcl-2, and apoptosis in 40 patients with uterine cervix carcinoma during radiotherapy, indicating a positive apoptosis rate, increased p53 and Fas expression, and significantly decreased Bcl-2 after radiotherapy. Several studies have demonstrated that apoptosis induced by chemotherapy involved the Fas/FasL system (Bewick et al., 2001; Shimizu et al., 2005; Naumnik et al., 2007; Chaudhry et al., 2008). Cytotoxic drugs lead to increasing FasL expression, which triggers tumor cells into apoptosis by binding Fas (Eichhorst et al., 2001). Debatin and Krammer (2004) expressed a different view that the Fas/FasL system may amplify the mitochondrial pathway to induce apoptosis. Many studies have confirmed that chemotherapy decreases serum sFas (Shimizu et al., 2005; Naumnik et al., 2007; Chaudhry et al., 2008), which relieves the inhibition of Fas/FasL system, and amplifies the apoptotic signal leading to tumor shrinkage.
The concentration of sFas in colorectal cancer correlated with the clinicopathological stage and treatment response (Kushlinskii et al., 2001). Abbasova et al. (2009) found that the sFas median concentration increased with tumor progression, and sFas levels showed significant differences between cases of regional metastases and no metastases in colorectal cancer. In the present study, the sFas levels before and after treatment in those with LAURC were higher than those in healthy controls. Furthermore, the sFas level after treatment was significantly lower than that before treatment. This significant change may be a result of concurrent chemoradiotherapy. In order to further investigate the prognostic value of sFas, according to mean sFas levels before and after treatments, subjects were divided into four groups, and followed up for 16 months. As a result, the low sFas level groups before and after treatment both had significant differences compared with the high sFas level groups in their one-year survival rates. In other words, low sFas levels were associated with a better prognosis.
In conclusion, concurrent chemoradiotherapy can decrease serum sFas level in LAURC patients. The monitoring of sFas may provide prognostic information for these patients.
References
- 1.Abbasova SG, Vysotskii MM, Ovchinnikova LK, Obusheva MN, Digaeva MA, Britvin TA, Bahoeva KA, Karabekova ZK, Kazantzeva IA, Mamedov UR, et al. Cancer and soluble Fas. Bull Exp Biol Med. 2009;148(4):638–642. doi: 10.1007/s10517-010-0784-8. [DOI] [PubMed] [Google Scholar]
- 2.Bewick M, Conlon M, Parissenti AM, Lee H, Zhang L, Glück S, Lafrenie RM. Soluble Fas (CD95) is a prognostic factor in patients with metastatic breast cancer undergoing high-dose chemotherapy and autologous stem cell transplantation. J Hematother Stem Cell Res. 2001;10(6):759–768. doi: 10.1089/152581601317210854. [DOI] [PubMed] [Google Scholar]
- 3.Boroumand-Noughabi S, Sima HR, Ghaffarzadehgan K, Jafarzadeh M, Raziee HR, Hosseinnezhad H, Moaven O, Rajabi-Mashhadi MT, Azarian AA, Mashhadinejad M, et al. Soluble Fas might serve as a diagnostic tool for gastric adenocarcinoma. BMC Cancer. 2010;10(1):275. doi: 10.1186/1471-2407-10-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosset JF, Calais G, Daban A, Berger C, Radosevic-Jelic L, Maingon P, Bardet E, Pierart M, Briffaux A EORTC Radiotherapy Group. Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: assessment of acute toxicity and treatment compliance. Report of the 22921 randomised trial conducted by the EORTC Radiotherapy Group. Eur J Cancer. 2004;40(2):219–224. doi: 10.1016/j.ejca.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Cascino I, Fiucci G, Papoff G, Ruberti G. Three functional soluble forms of the human apoptosis-inducing Fas molecule are produced by alternative splicing. J Immunol. 1995;154(6):2706–2713. [PubMed] [Google Scholar]
- 6.Chaudhry P, Srinivasan R, Patel FD, Gopalan S, Majumdar S. Serum soluble Fas levels and prediction of response to platinum-based chemotherapy in epithelial ovarian cancer. Int J Cancer. 2008;122(8):1716–1721. doi: 10.1002/ijc.23213. [DOI] [PubMed] [Google Scholar]
- 7.Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23(16):2950–2966. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- 8.Eichhorst ST, Müerköster S, Weigand MA, Krammer PH. The chemotherapeutic drug 5-fluorouracil induces apoptosis in mouse thymocytes in vivo via activation of the CD95 (APO-1/Fas) system. Cancer Res. 2001;61(1):243–248. [PubMed] [Google Scholar]
- 9.Gratas C, Tohma Y, Barnas C, Taniere P, Hainaut P, Ohgaki H. Up-regulation of Fas (APO-1/CD95) ligand and down-regulation of Fas expression in human esophageal cancer. Cancer Res. 1998;58(10):2057–2062. [PubMed] [Google Scholar]
- 10.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270(5239):1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 11.Havenga K, Enker WE, Norstein J, Moriya Y, Heald RJ, van Houwelingen HC, van de Velde CJ. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol. 1999;25(4):368–374. doi: 10.1053/ejso.1999.0659. [DOI] [PubMed] [Google Scholar]
- 12.Jodo S, Kobayashi S, Nakajima Y, Matsunaga T, Nakayama N, Ogura N, Kayagaki N, Okumura K, Koike T. Elevated serum levels of soluble Fas/APO-1 (CD95) in patients with hepatocellular carcinoma. Clin Exp Immunol. 1998;112(2):166–171. doi: 10.1046/j.1365-2249.1998.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kachnic LA. Adjuvant chemoradiation for localized rectal cancer: current trends and future directions. Gastrointest Cancer Res. 2007;1(4 Suppl. 2):S64–S72. [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura K, Gelmann EP. Tumor necrosis factor-alpha and Fas activate complementary Fas-associated death domain-dependent pathways that enhance apoptosis induced by gamma-irradiation. J Biol Chem. 2000;275(12):8610–8617. doi: 10.1074/jbc.275.12.8610. [DOI] [PubMed] [Google Scholar]
- 15.Klautke G, Feyerherd P, Ludwig K, Prall F, Foitzik T, Fietkau R. Intensified concurrent chemoradiotherapy with 5-fluorouracil and irinotecan as neoadjuvant treatment in patients with locally advanced rectal cancer. Br J Cancer. 2005;92(7):1215–1220. doi: 10.1038/sj.bjc.6602492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kushlinskii NE, Britvin TA, Abbasova SG, Perevoshchikov AG, Prorokov VV, Kostanyan IA, Knysh VI, Lipkin VM. Soluble Fas antigen in the serum of patients with colon cancer. Bull Exp Biol Med. 2001;131(4):361–363. doi: 10.1023/A:1017908320634. [DOI] [PubMed] [Google Scholar]
- 17.Liang QL, Pan DC, Yin ZM, Liu GX, Yang Q, Xie JR, Cai LZ, Fu YW. Clinical value of serum soluble Apo-1/Fas for predicting biological behaviors and prognosis of gastric carcinoma. Ai Zheng. 2002;21(2):174–176. (in Chinese) [PubMed] [Google Scholar]
- 18.Liang QL, Wang BR, Li GH. DcR3 and survivin are highly expressed in colorectal carcinoma and closely correlated to its clinicopathologic parameters. J Zhejiang Univ-Sci B. 2009;10(9):675–682. doi: 10.1631/jzus.B0920077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natoli G, Ianni A, Costanzo A, de Petrillo G, Ilari I, Chirillo P, Balsano C, Levrero M. Resistance to Fas-mediated apoptosis in human hepatoma cells. Oncogene. 1995;11(6):1157–1164. [PubMed] [Google Scholar]
- 20.Naumnik W, Izycki T, Ossolinska M, Chyczewska E. Serum levels of sFas and sFasL during chemotherapy of lung cancer. Exp Oncol. 2007;29(2):132–136. [PubMed] [Google Scholar]
- 21.Nesbakken A, Nygaard K, Westerheim O, Mala T, Lunde OC. Local recurrence after mesorectal excision for rectal cancer. Eur J Surg Oncol. 2002;28(2):126–134. doi: 10.1053/ejso.2001.1231. [DOI] [PubMed] [Google Scholar]
- 22.Pan G, Ahn EY, Chen Y, Feng G, Reddy V, Jhala NC, McDonald JM. Reciprocal co-expression of Fas and Fas ligand in human cholangiocarcinoma. Int J Oncol. 2007;31(4):843–850. [PubMed] [Google Scholar]
- 23.Petak I, Tillman DM, Harwood FG, Mihalik R, Houghton JA. Fas-dependent and independent mechanisms of cell death following DNA damage in human colon carcinoma cells. Cancer Res. 2000;60(10):2643–2650. [PubMed] [Google Scholar]
- 24.Shimizu M, Kondo M, Ito Y, Kume H, Suzuki R, Yamaki K. Soluble Fas and Fas ligand provide new information on metastasis and response to chemotherapy in SCLC patients. Cancer Detect Prev. 2005;29(2):175–180. doi: 10.1016/j.cdp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Spindler KL, Nielsen JN, Lindebjerg J, Brandslund I, Jakobsen A. Prediction of response to chemoradiation in rectal cancer by a gene polymorphism in the epidermal growth factor receptor promoter region. Int J Radiat Oncol Biol Phys. 2006;66(2):500–504. doi: 10.1016/j.ijrobp.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 26.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand a novel member of the tumor necrosis factor family. Cell. 1993;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-L. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Q, Han SX, Li MZ, Su BS, Ma JL. The expression of Fas, p53, Bcl-2 and cervix carcinoma cell apoptosis during radiotherapy. Modern Oncol. 2006;14(6):730–732. (in Chinese) [Google Scholar]