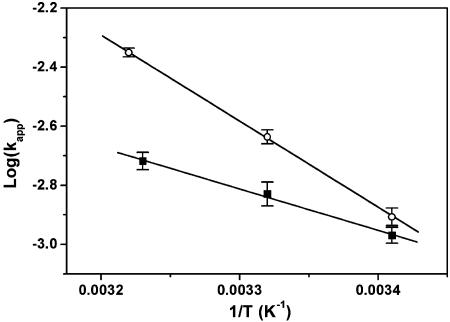
Fig. 5.
Arrhenius plot of the observed single-molecule kinetic rates in 100 mM NaCl (○) and in 100 mM KCl (▪). The derived activation energies and entropies are 14.9 ± 0.2 kcal·mol–1 and –23.0 ± 0.8 cal·mol–1 and 6.4 ± 0.4 kcal·mol–1·K–1 and –52.3 ± 1.4 cal·mol–1·K–1 for sodium- and potassium-containing buffer, respectively.