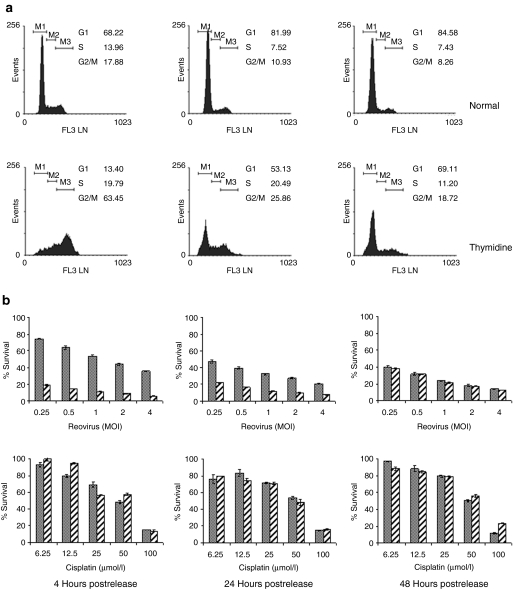
Figure 3.
Synchronization of B16.F10 cells by double thymidine block. Following overnight adherence, B16.F10 cells were cultured for 18 hours in medium supplemented with 2 mmol/l thymidine. After a 9-hour release period in nonsupplemented medium, the cells were cultured for a further 17 hours in 2 mmol/l thymidine-supplemented medium. Incubation continued in nonsupplemented medium for the duration indicated, at which point cells were harvested for analysis. Untreated cells were used as normal cycling control populations. (a) Cells were harvested at the time points indicated postrelease, fixed, and stained with propidium iodide before fluorescence-activated cell-sorting analysis to determine DNA content. (b) At the times indicated, aliquots of 7.5 × 103 treated (hatched bars) or normal cycling (solid bars) cells were infected with reovirus at the MOI indicated or cisplatin at the concentrations indicated. After 48 hours incubation, cell survival was estimated by MTS [3-(4,5-dimethylthiazol-2-yl)- 5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)] assay. At 4 hours postrelease, the thymidine-synchronized cells were more sensitive to reovirus-induced cell death than normal cycling cells (P < 0.0001), but as the cells returned to normal distribution, this effect was lost and there was no difference in survival by 48 hours (P = 0.5933). There was a small survival advantage in thymidine-synchronized cells exposed to cisplatin 4 hours postrelease (P = 0.0040), but this also diminished over time (P = 0.01638 and P = 0.3563). Data shown are mean survival ± SEM. MOI, multiplicity of infection.