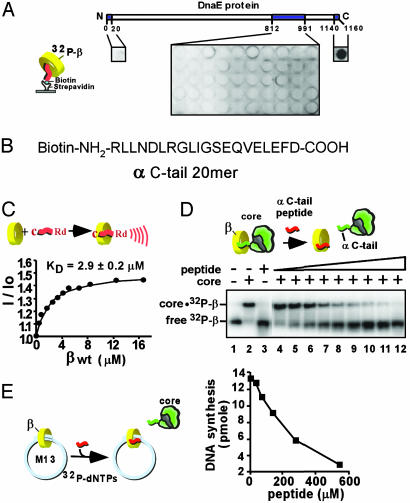
Fig. 2.
The α C terminus of Pol III core binds to the β clamp. (A) N-terminal biotinylated 20-mer peptides corresponding to an internal region and the N and C termini of α (as indicated by shading) were attached to streptavidin-coated microtiter plates and assayed for binding to [32P]β.(B) Sequence corresponding to the C-terminal 20 residues of α. (C) Use of rhodamine-labeled α C-tail 20-mer to measure the KD of interaction with the β clamp by fluorescence. (D) Native PAGE mobility shift assay of [32P]β interaction with core. Lanes 4–12 contain α C-tail peptide at either 2.5, 4.8, 9.5, 19, 38, 76, 153, 306, or 613 μM. (E) The α C-tail peptide inhibits β-dependent replication of singly primed M13mp18 ssDNA catalyzed by core polymerase, β, and γ complex.