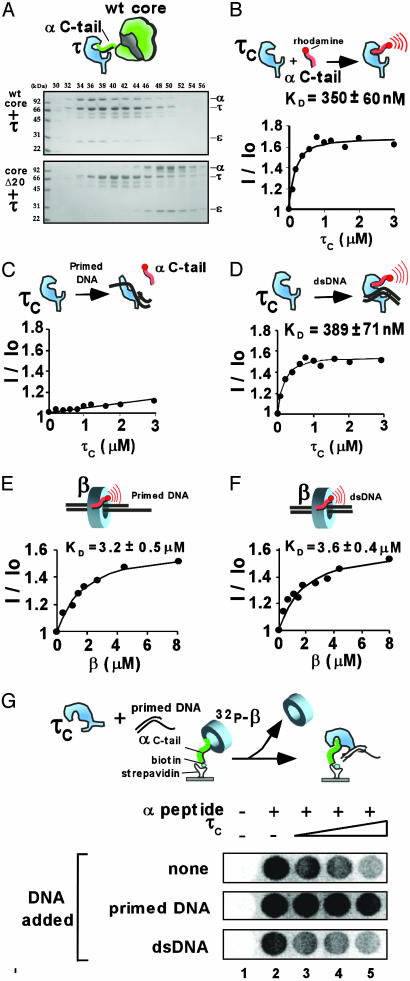
Fig. 4.
The τ subunit binds the polymerase C-tail, and the strength is modulated by DNA. (A) Interaction of τ with core (Upper gel) and lack thereof by using core Δ20 (Lower gel) as analyzed by gel filtration using a Superose 12 column. The affinity of τc for the N-terminal rhodamine labeled α C-tail 20-mer peptide was determined by fluorescence by using no DNA (B), primed DNA (C), or duplex DNA (D). τ was 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.6, 2.0, or 3.0 μM. E and F demonstrate that DNA has little effect on the affinity of β for the α C-tail peptide. Reactions were performed as in C and D, respectively, except that β (at 0, 0.3, 0.7, 1.1, 1.5, 2.6, 3.5, 4.5, or 8 μM) replaced τc. (G) To determine whether τ and β compete for the α C-tail, biotinylated α C-tail 20-mer was immobilized in wells of a strepavidin-coated 96-well plate and then incubated with [32P]β (44 nM) and τ (0, 0.8, 1.75, or 3.5 μM) before washing the wells and examining them by PhosphorImager. The synthetic primed template abrogates the ability of τ to displace [32P]β from the α C-tail (Middle). Double-stranded DNA has little effect on the competition (Bottom).