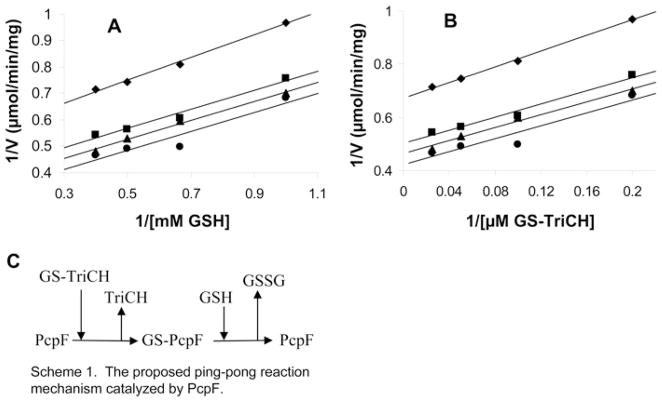
Figure 4. Double reciprocal plots of initial velocities of PcpF against substrate concentrations.
A GS-TriCH enzymatic assay was performed [a coupled assay of PcpF (23 μg · ml−1 at 2.66 μmol · min−1 · mg−1) and glutathione reductase (2.7 μg · ml−1 at 168 μmol · min−1 · mg−1)]. Results are means of two measurements. (A) Enzyme activity was plotted against various concentrations of GSH with four sets of constant concentrations of GS-TriCH. (B) Enzyme activity was plotted against various concentrations of GS-TriCH with four sets of constant concentrations of GSH. (C) Scheme of deduced Ping Pong mechanism of PcpF from kinetic analysis. The GSH in the scheme can be replaced by DTT, L-cysteine and 2-mercaptoethanol.