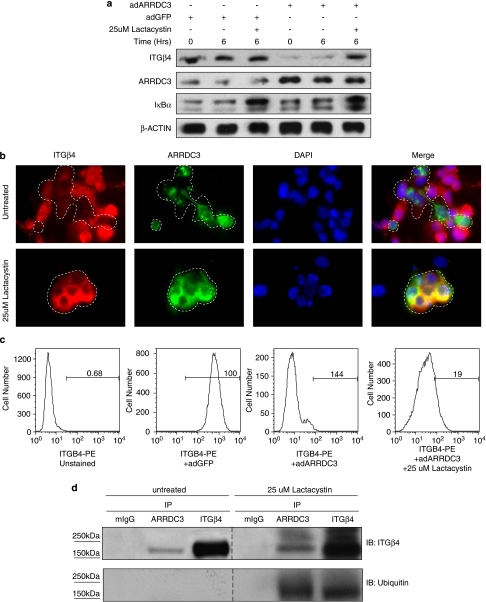
Figure 6.
In breast cancer cells, ARRDC3 directly interacts with ubiquitinated ITGβ4 and negatively regulates protein levels in a mechanism dependent on the proteosome. (a, b) Cells can maintain high levels of ITGβ4 after ARRDC3 overexpression if treated with proteosome inhibitor Lactacystin for 6 h. (a) Western blot analysis shows that ITGβ4 levels after ARRDC3 overexpression is restored when the proteosome is inhibited. Inhibition of the proteosome is demonstrated by the accumulation of IκBα. (b) Immunofluorescence demonstrates that ITGβ4 levels are retained after ARRDC3 overexpression if cells are treated with proteosome inhibitor. Cells positive for adARRDC3 (green fluorescent protein (GFP+)) are outlined with a dashed line. (c) ARRDC3 causes a complete removal of ITGβ4 from the cell surface that is partially rescued with proteosome inhibition. The cell surface of live cells were then stained with an ITGβ4 antibody and analyzed by flow cytometry. Uninfected GFP-negative cells were gated out before ITGβ4 levels were examined. (d) Pretreatment of MDA-MB-231 cells with proteosome inhibitor lactacystin before the endogenous coimmunoprecipitation with antibodies against ARRDC3 and ITGβ4 enriches ubiquitinated forms of ITGβ4. Bands seen in the ubiquitin immunoblots were identical in size to ITGβ4 bands. Dotted line indicates noncontiguous lanes from the same film.