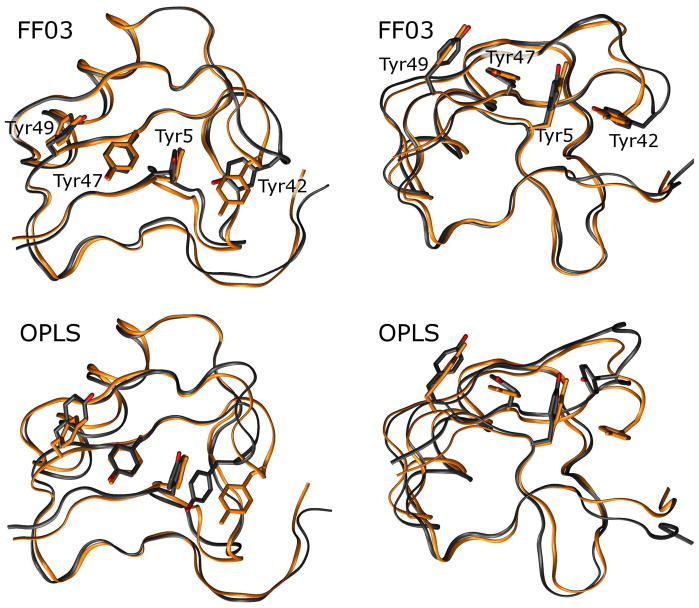
Figure 7. Distortion of the residues 39–43 hairpin can occur with loss of interactions between Tyr42 and Tyr5.
While all force fields seem to allow the residues 39 to 43 β-hairpin turn to become distorted, the FF03 and OPLS force fields allow the associated Tyr42 side chain to adopt non-native conformations in which it breaks its favorable T-stacking interaction with Tyr5. Above, the X-ray structure is shown in orange while a representative monomer from a simulation performed with each force field is shown in black; structures are superimposed by quaternion alignment of backbone atoms. None of the force fields contains explicit terms for the T-stacking effect, though it apparently stabilizes four tyrosine side chains in the X-ray structure. More importantly, the FF99SB and CHARMM force fields maintain the interaction between tyrosine side chains; it is not certain whether the FF99SB and CHARMM force fields have superior pairwise terms to stabilize the nonbonded interactions between side-chains, or if they simply have superior backbone dihedral terms which prevent the side chains from exploring non-native conformations. Future studies with restrained simulations which isolate backbone and side chain degrees of freedom may provide these answers.