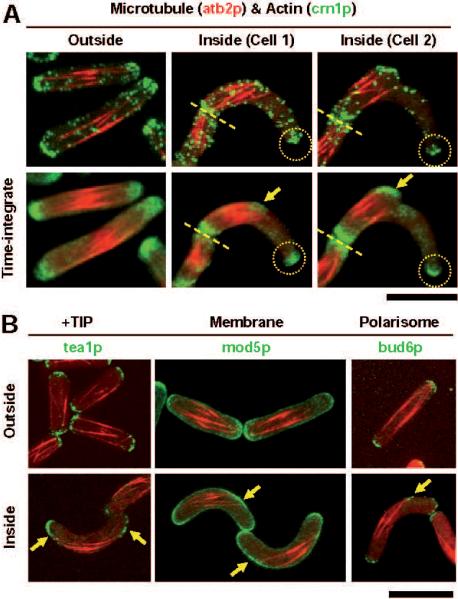
Figure 2. Cell shape-induced microtubule reorganization leads to new cell polarization sites.
A. Wildtype fission yeast cells expressing mCherry-atb2p (tubulin) and crn1p-GFP (a marker for actin patches) (PT.832). Top row shows single time point 2-color merged images of cells. Second row shows 2-color time-integrated images where 60 time points, representing 1 hour of cell growth, have been summed to show cumulative intensities over time. Cells inside the channel (cell 1) contain rigid microtubule bundles which can no longer extend to both cell tips. Instead, the microtubules reach one tip of the cell and make frequent and sustained contact with the cell cortex (yellow arrows) some distance away from the second tip (yellow dashed circle). Time-integration shows that actin patches accumulates at the ectopic site of microtubule and membrane contact. With longer growth times inside channels (cell 2) this site shows clearer localization of actin (yellow arrow) and the initiation of a new ectopic cell tip, making a branched wildtype cell. The old cell tip continues to be enriched in actin and grow in the absence of microtubule contact (yellow dashed circle) (n=12; control cells n>30).
B. Wildtype cells expressing mCherry-atb2p (tubulin) and proteins of the +TIP (tea1p-GFP) (PT.826), cell membrane (mod5p-GFP) (PT.907), or polarisome (bud6p-GFP) cytoskeleton (PT.853). Outside channels, cell tip proteins show clear symmetry in localization at the bipodal tips of the rod-shaped cells (n>30). Inside curved channels, tip protein symmetry is broken. Consistent with the reorganization of microtubules to the convex cell surface and microtubule contact at new ectopic membrane sites, all tip proteins show relocalization to the ectopic site (yellow arrows) while maintaining localization at the old cell tips (n=19). Bar, 10 μm.