Figure 1.
Microtubule organization in fission yeast.
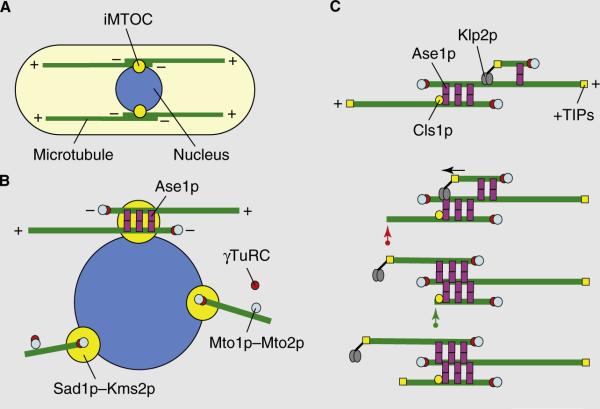
(A) A typical fission yeast cell has between three and five dynamic microtubule bundles organized along the long axis of the cell that are organized by iMTOCs into antiparallel bundles with minus ends overlapping at the middle of the cell and plus ends facing and interacting with the cell tips. Two complementary modes of microtubule organization are presented in (B) and (C). (B) In the first model, iMTOCs are tethered to the nuclear membrane. The Mto1p–Mto2p complex, a component of the iMTOC, recruits γ-TuRCs which nucleate microtubules. Microtubule polymers are then bundled into an antiparallel configuration by Ase1p. (C) In the second model, new microtubules nucleate on pre-existing microtubules. The Mto1p–Mto2p complex recruits γ-TuRCs to the lattice of a pre-existing microtubule. Ase1p stabilizes the antiparallel configuration between new and old microtubules. The kinesin Klp2p slides the new microtubule to the minus end of the old microtubule (marked by the arrow), establishing an antiparallel bundle. Microtubule length is regulated by +TIP proteins and the rescue factor Cls1p/Peg1p. A growing microtubule can exhibit catastrophe and shrinkage (red arrow). It can then be rescued by Cls1p/Peg1p at the iMTOC and re-grow (green arrow).