Table 1.
Initial studies at determining optimal conditions for synthesis of the O6-(benzotriazol-1-yl)guanosine analogues 3 and 4a
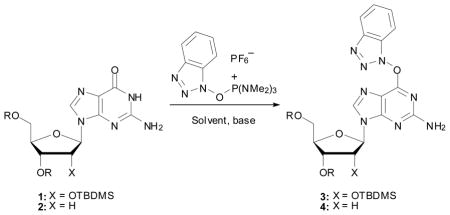 | ||||||
|---|---|---|---|---|---|---|
| Entry | Substrate | Solvent | Base | Temp | Time | Yieldb |
| 1 | 1 | DMSO | i-Pr2NEt | 55 °C | 24 h | 54% |
| 2 | 1 | THF | i-Pr2NEt | rt | 96 h | NRc |
| 3 | 1 | THF | DBU | rt | 4 h | 45% |
| 4 | 1 | CH3CN | DBU | rt | 2 h | 65% |
| 5 | 2 | CH3CN | DBU | rt | 1 h | 85% |
Reactions were performed using 2.0 molar equiv each of BOP and DBU at ~0.1 M nucleoside concentration.
Where reported, yield is of isolated and purified products.
No reaction was observed and only 1 was present.
