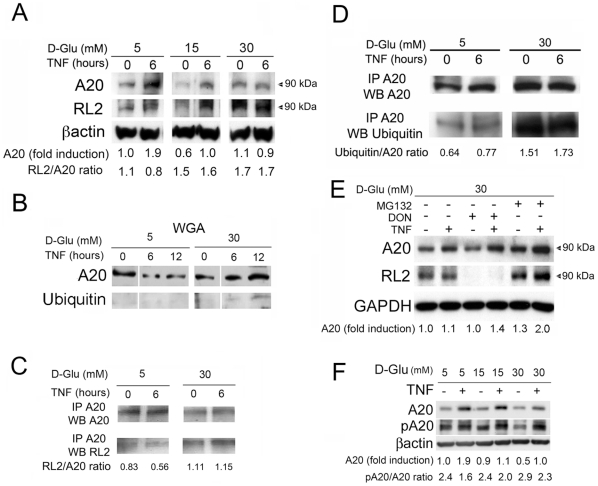
Figure 5. O-GlcNAcylation and ubiquitination of A20 modulate its expression.
(A) WB analysis of total A20 and co-immunoblotted, overlapping GlcNAc-A20 (RL-2) in SMC cultured in 5, 15 and 30 mM of D-glucose (D-Glu), and treated or not with TNF for 6 h. β-actin was used as a control for loading. (B) WB analysis of WGA captured proteins from SMC cultured in 5 and 30 mM D-Glu demonstrate the presence of glycosylated (GlcNAcA20), and co-immunoblotted, overlapping, ubiquitinated A20 (Ub-A20). (C) WB analysis of cell lysates immunoprecipitated with the A20 antibody from SMC cultured in 5 and 30 mM of D-Glu and treated or not with TNF for 6 h, and analyzed WB for total A20 and GlcNAc-A20 using the RL2 antibody demonstrate increased GlcNAc-A20 in high glucose medium. (D) WB analysis of cell lysates immunoprecipitated with the A20 antibody from SMC cultured in 5 and 30 mM D-Glu and treated or not with TNF for 6 h, and analyzed by WB for total and Ub-A20 demonstrate increased Ub-A20 in high glucose medium. (E) WB analysis of total and overlapping GlcNAc-A20 (RL-2) in SMC cultured in 30 mM D-Glu and treated with DON (prior to TNF) or MG132 (after TNF). (F) WB analysis of total and phospho-A20 in SMC cultured in 5, 15 and 30 mM D-Glu and treated with TNF for 6 h demonstrated that relative phosphorylation levels of A20 (pA20) were not decreased by high glucose, despite decreased TNF-mediated upregulation of A20 protein in cells cultured in high glucose. GAPDH or βactin was checked as a loading control to quantify A20 expression by densitometry. Corrected A20 fold-inductions are listed below the WB. RL2/A20 and Ubiquitin/A20 ratios were also calculated by densitometry. Data shown in A, C, D, and E are representative of 3 independent experiments. Data shown in B and F are representative of 2 independent experiments.