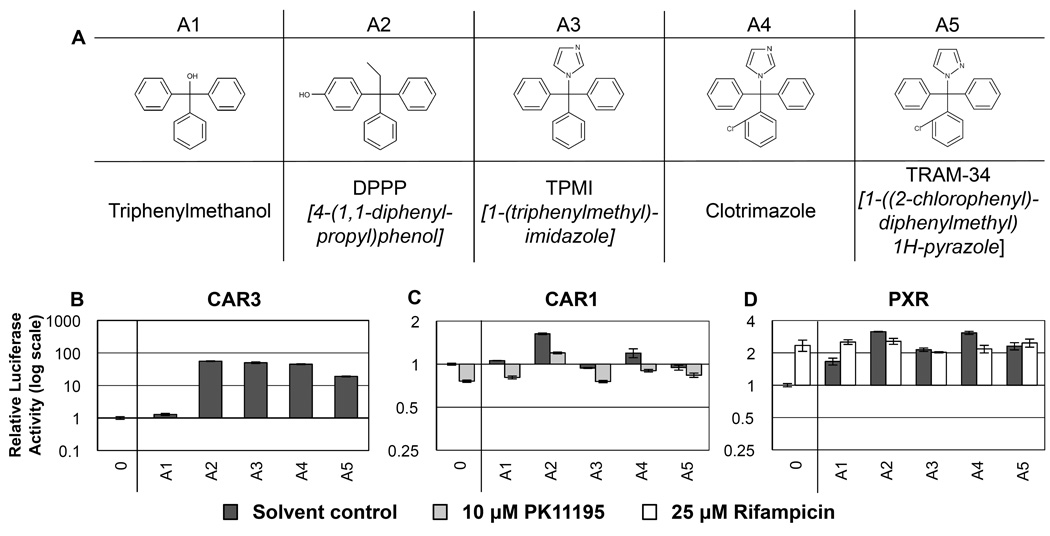
Figure 1. RQSAR for group A compounds - Triphenyls.
(A) 2D structures for group A triphenyls. (B) CAR3-driven relative luciferase activity, (C) CAR1, and (D) PXR treated with 10 µM test compound, plotted using log scales. 0 = DMSO solvent control. Cotreatments were PK11195 (10 µM) for CAR1 and rifampicin (25µM) for PXR. (n=4 except for B: A2, A4 and A5 n=8. C: A1 n=3).