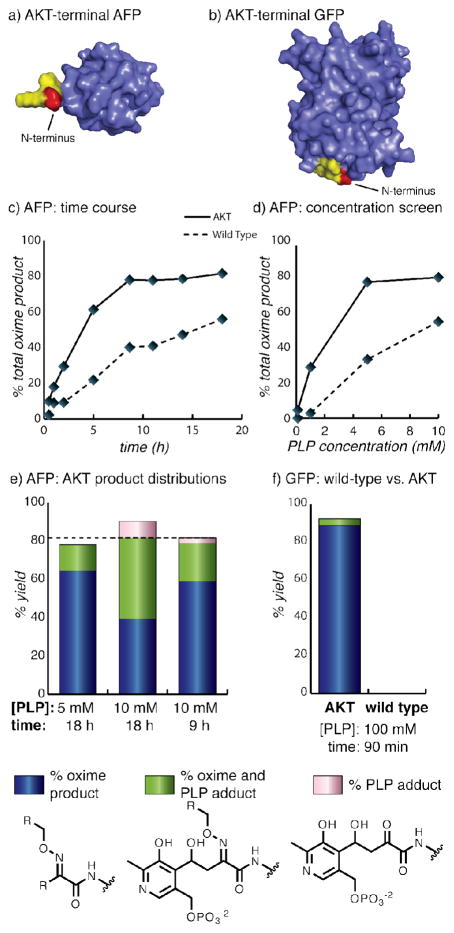
Figure 6.
The performance of wild-type and AKT-terminal sequences were compared for the transamination of two protein substrates. The positions of the new termini are shown for (a) an “Antifreeze” Protein (AFP) and (b) GFP. The Ala residue is in red and the Lys-Thr portion is in yellow. (c) The transamination of the AFP mutant showed that the AKT sequence outperformed the wild-type terminus (GNQ) at every time point analyzed. (d) The AKT-terminal AFP also outperformed the wild-type sequence at different concentrations of PLP. (e) The product distribution of the AKT-terminal AFP mutant shows that maximum oxime yield (blue plus green) and minimum PLP adduct (green plus pink) could be achieved by optimizing the reaction conditions. The dotted line marks the highest total oxime yield acheived, demonstrating that the strategies that decreased byproduct formation did not sacrifice overall yield. (f) Using the strategy of a high concentration of PLP for a short reaction time, conditions were found that gave a high oxime yield with minimal byproducts for AKT-terminal GFP in only 90 minutes. The wild type GFP mutant achieved no modification under the same conditions, highlighting the enhanced reactivity conferred by the AKT sequence.