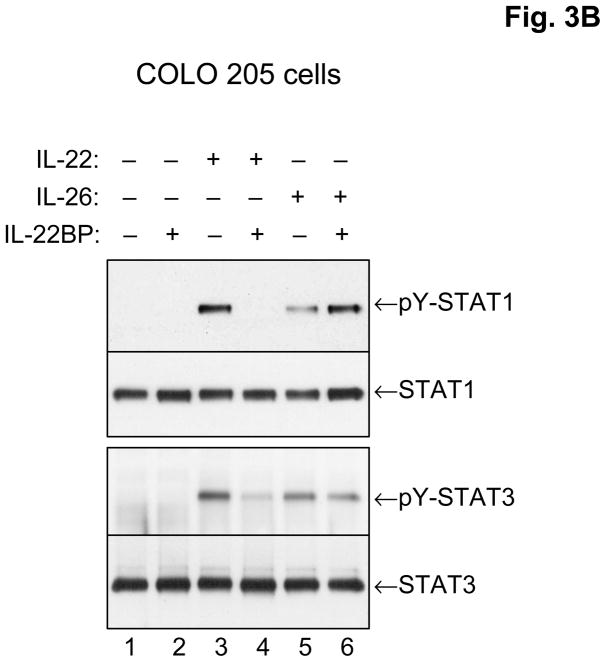
Figure 3.
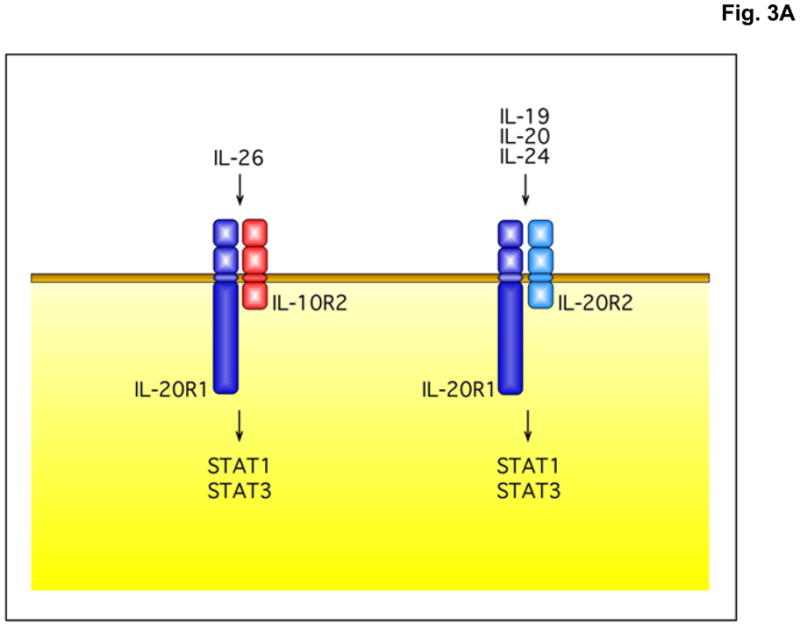
The IL-26 receptor complex. A. The IL-26 receptor complex is composed of two polypeptide chains: the ligand-binding chain, IL-20R1, and the accessory receptor chain, IL-10R2. IL-26 binds initially to IL-20R1, and then rapidly recruits the IL-10R2 chain to complete assembly of the active receptor complex. Ligand-induced heterodimerization of these receptor chains initiates a signal transduction cascade that results in the activation and nuclear translocation of STAT1 and STAT3. IL-20R1 can also dimerize with another class II cytokine receptor, IL-20R2, to generate receptors for IL-19, IL-20 and IL-24. B. IL-26 induces tyrosine phosphorylation of STAT1 and STAT3 in the human colorectal carcinoma cell line, COLO 205. COLO 205 cells were incubated with IL-22 or IL-26 (100 ng/mL) in the presence or absence of recombinant human IL-22 binding protein (IL-22BP) for 30 minutes at 37°C. At the end of this incubation period, whole cell lysates were prepared and analyzed by western blotting with antibodies specific for tyrosine-phosphorylated STAT1 or tyrosine-phosphorylated-STAT3 and total STAT1 and total STAT3.