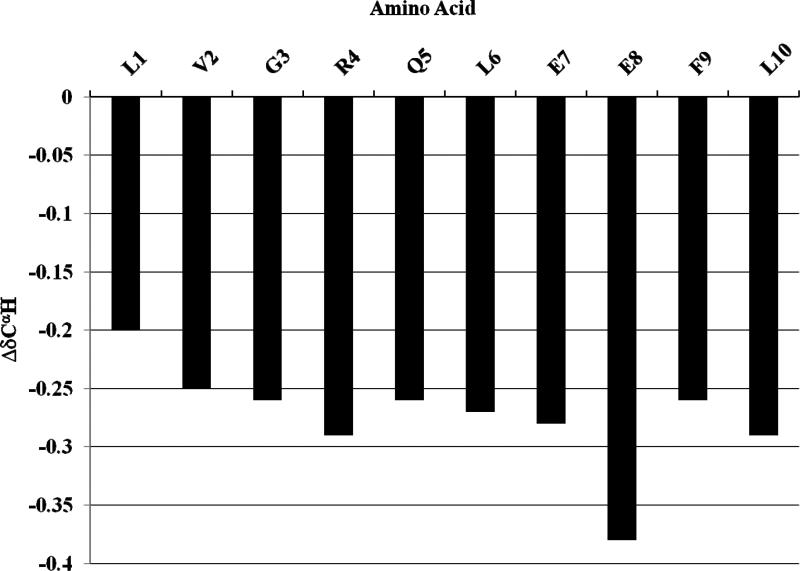
Figure 7. CαH proton chemical shifts indicate predominantly α helical secondary structure of [113-122]apoJ in the presence of DPC micelle.
A plot of the ΔδCαH (δobserved-δcoil) chemical shift for all the residues in [113-122]apoJ. Note that the CαH protons show an upfield shift compared to the corresponding coil values, indicating α-helical structure. Random coil chemical shifts used were those reported in: Wuthrich, K. (1986) NMR of Proteins and Nucleic Acids, John Wiley & Sons, Inc., New York