Figure 4.
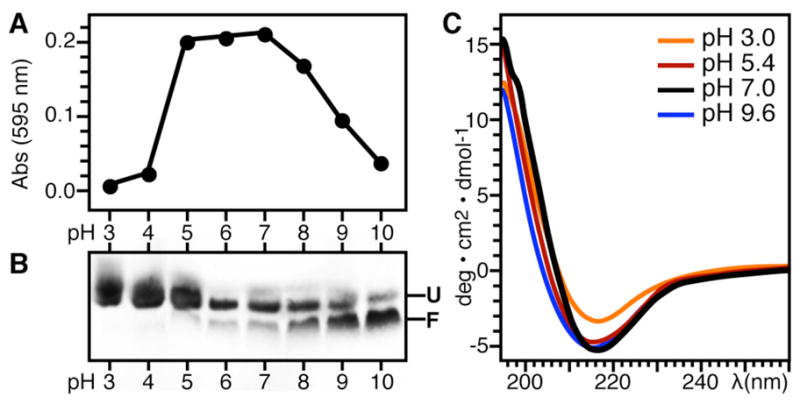
Ail adopts a β-sheet structure and refolding is assisted by high pH. (A) Protein aggregation at pH 3–10 monitored by visible light absorbance at 595 nm. (B) Corresponding SDS-PAGE showing Ail refolding in DHPC for each light absorbance measurement over the pH range from 3 to 10. The refolding reactions contained 70 mM DHPC, 100 mM KCl, and 600 mM Arginine, plus 20 mM of the appropriate buffer (Table 2). Folded (F) and unfolded (U) Ail migrate as different bands. Samples were loaded without boiling. (C) CD spectra of Ail refolded in DPC at pH 9.6 and then adjusted to pH 7-3 by the addition of HCl.
