Table 1. Fraction of atoms that are charged (crg), polar (pol), and apolar (apl) based on the total atom number (f0), surface atom number (fS), excess fraction of charged atoms on the surface, and depletion of charged atoms in the interior.
| Protein |
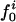 * *
|
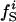 † †
|
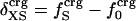 † †
|
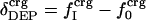 † †
|
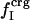 ‡ ‡
|
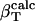 ‡ ‡
|
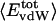 ‡ ‡
|
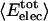 § §
|
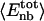 ¶ ¶
|
|---|---|---|---|---|---|---|---|---|---|
| Trypsin | 5.16 | 3.5 | 34.9 | 61.7 | 8.6 | 43.8 | 47.7 | 5.1 | -1.8 |
| α-Lactalbumin | 12.4 | 6.4 | 31.1 | 62.5 | 15.0 | 33.3 | 51.7 | 8.5 | -3.2 |
| Hen egg-white lysozyme | 7.73 | 8.0 | 32.5 | 59.5 | 14.7 | 39.2 | 46.1 | 6.7 | -2.5 |
| RNase A Sixteen-protein set∥ | 5.48 | 6.1 | 34.6 | 59.3 | 11.0 | 41.4 | 47.5 | 4.9 | -2.0 |
| Mean | 5.6 | 31.9 | 62.4 | 12.2 | 37.4 | 50.4 | |||
| SD | 1.8 | 2.2 | 1.5 | 4.0 | 5.0 | 2.7 | |||
| SD/mean, % | 32.0 | 6.8 | 2.4 | 33.0 | 13.3 | 5.3 |
βTexp from ref. 19.
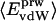 is the ratio between the overall number of atoms of type i, where i = (crg, pol, apl), and the total number of atoms in the protein (details in Methods).
is the ratio between the overall number of atoms of type i, where i = (crg, pol, apl), and the total number of atoms in the protein (details in Methods).
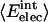 is the ratio between the number of atoms of type i on the protein surface and the total number of atoms on the surface.
is the ratio between the number of atoms of type i on the protein surface and the total number of atoms on the surface.
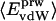 .
.
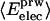 where
where 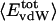 is the fractional number of charged atoms buried inside the protein.
is the fractional number of charged atoms buried inside the protein.
Proteins used in this study were (with Protein Data Bank ID codes) ovalbumin (1OVA), pepsin (1AM5), chymotrypsinogen A (1EX3), α-chymotrypsin (4CHA), trypsinogen (1TGN), myoglobin (1MDN), subtilisin BPN (1UBN), ovomucoid (1TUS), carbonic anhydrase (1AVN), β-lactoglobulin (1B8E), peroxydase (1A20), cytochrome c (1A7V), trypsin inhibitor (1BP1), hemoglobin (1A9W), and anionic trypsin (1ANE).
