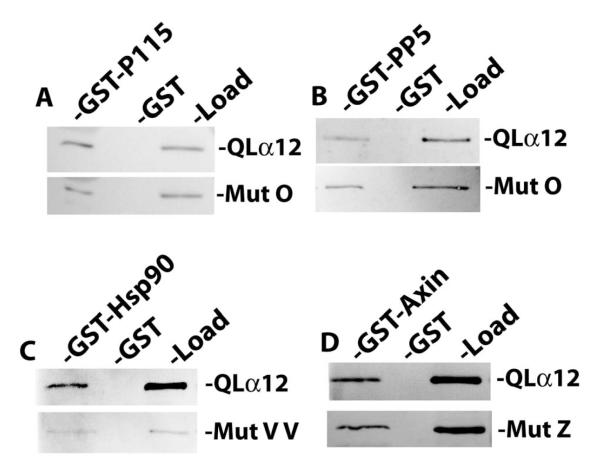
Figure 2. Gα12 mutations uncoupled from PC1 binding interact normally with other Gα12 targets.
For all panels, the indicated NAAIRS mutants and QLα12 were expressed in HEK293 cells and examined in pulldown experiments as described in 2.4. (A, B) Mutant O was tested for in vitro interaction with GST fusions of p115RGS (GST-p115) and protein phosphatase-5 (GST-PP5). For each pulldown condition the binding of the mutant was compared to results for QLα12 analyzed in parallel. (C.) Mutant Z and QLα12 were tested for interaction with a GST fusion of the RGS domain of axin (GST-Axin). (D.) Mutant VV and QLα12 were tested for binding to GST-Hsp90. For all panels, the band intensities were quantified using Carestream Molecular Imaging software, and the ratio of pulldown-to-load for each mutant was compared to the pulldown-to-load ratio for QLα12 tested in parallel.