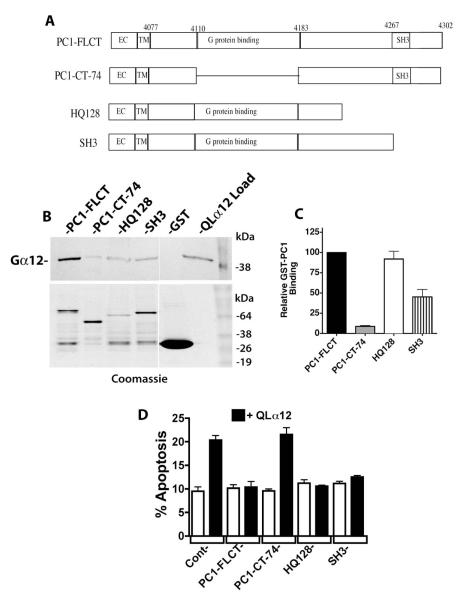
Figure 4. PC1 C-terminus requires a minimal G protein binding domain for Gα12 interaction and regulation of apoptosis.
(A) Diagram of PC1 C-terminal deletions. Mutations were engineered as described in 2.2 within a GST fusion of PC1 (used for pulldown experiments; panels B and C) and a CD16/CD7/PC1 fusion protein (used for transient cellular expression and determination of apoptosis; panel D). (B) GST pulldowns of QLα12 using PC1 C-terminal deletion mutations. Top panel is a Western blot showing the intensity of QLα12 precipitated by the indicated GST-PC1 constructs, and lower panel shows abundance of each GST-PC1 fusion protein within the precipitated sample. (C) Quantification of pulldown results. Band intensity values for precipitated QLα12 were normalized to the band intensity of each GST-PC1 protein using Carestream Molecular Imaging software and presented as a percent of the value for QLα12 precipitated by full length PC1 C-terminus (PC1-FLCT). (D) Inhibition of QLα12-stimulated apoptosis +/− PC1 C-terminal Mutants. HEK293 cells were transfected with a CD16/CD7/PC1 fusion protein with no deletion (FLCT) or the deletions CT-74, HQ128 or SH3 (see panel A). Constructs were transfected +/− a plasmid encoding QLα12 (white vs. black bars). Cells were analyzed for apoptosis by FACS as described in 2.7.