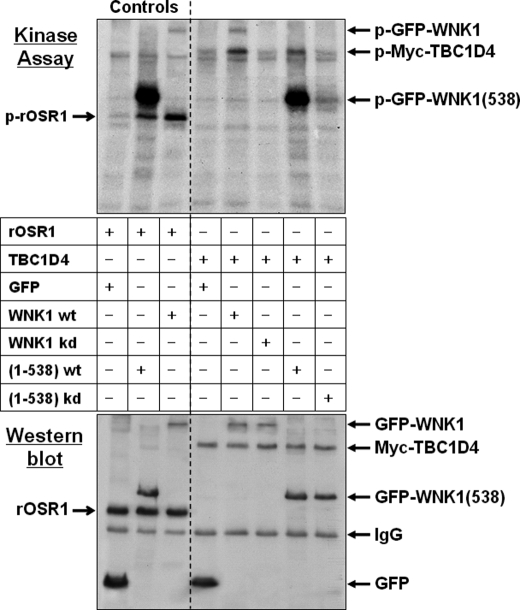
FIGURE 3.
WNK1 phosphorylates TBC1D4 in vitro. HEK293 cells were transfected with either GFP empty vector, GFP-WNK1, fragment GFP-WNK1(1–538), or the respective kinase-dead (kd) WNK1-K233M mutants. WNK1 was immunoprecipitated using anti-GFP antibodies and tested in an in vitro protein kinase assay. The substrates were either recombinant OSR1 a previously described physiological substrate (31) (Controls), or beads containing immunoprecipitated Myc-TBC1D4. After 30 min of incubation the samples were denatured by adding SDS sample buffer, then separated by gel electrophoresis and transferred to PVDF blotting membranes. Incorporated radioactive [32P]phosphate was detected by exposing the membranes for 24 h to x-ray films (Kinase Assay). Subsequently, the amounts of recombinant T7-OSR1 or immunoprecipitated proteins were documented by Western blot using anti-GFP and then reprobed with either anti-T7 or anti-Myc antibodies. Note that both WT full-length WNK1 and its fragment (1–538) autophosphorylate and also phosphorylate the physiological substrates OSR1 and TBC1D4. Whereas fragment 1–538 shows stronger autophosphorylation activity, possibly due to partial deletion of the inhibitory domain (63), WT full-length WNK1 has higher activity toward TBC1D4. Beads containing the respective kinase-dead mutants revealed no phosphorylation activity.