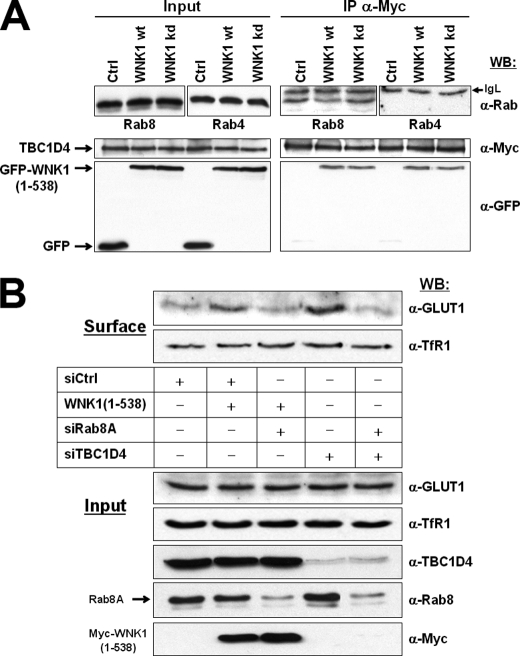
FIGURE 6.
Rab8A is required for GLUT1 expression downstream of WNK1. A, HEK293 cells were transfected as indicated and treated briefly with the mild reversible cross-linking reagent dithiobis(succinimidyl propionate) before lysis. Myc-TBC1D4 was immunoprecipitated and analyzed by Western blot (WB). Shown are the expression levels of GFP-WNK1(1–538), Myc-TBC1D4, and the endogenous Rab8 and Rab4 proteins in total cell lysates (left side panels, Input) as well as the immunoprecipitated fractions (right side panels, IP). Note that Rab8 but not Rab4 co-precipitated with TBC1D4 and that the amount decreased in the presence of WNK1, but increased in the presence of kinase-dead WNK1. B, HEK293 cells were transfected with control (siGFP) or Rab8A-specific (siRab8A) small interfering RNAs, either alone or in the presence of Myc-WNK1(1–538). In parallel, cells were transfected with TBC1D4-specific siRNAs (siTBC1D4), either alone or in combination with siRab8A. As described in Fig. 5A, the biotinylated cell surface proteins (Surface) and whole cell lysates (Input) were analyzed by Western blot to document the amounts of endogenous GLUT1, TfR1, TBC1D4, or Rab8, as well as of transfected Myc-WNK1(1–538). Note the successful depletion of TBC1D4 (lanes 4 and 5) and Rab8A (lanes 3 and 5). GLUT1 surface expression was promoted by either expression of WNK1 or depletion of TBC1D4 and the simultaneous knockdown of Rab8A impaired both effects. Under these conditions the surface levels of endogenous TfR1 remained unchanged.