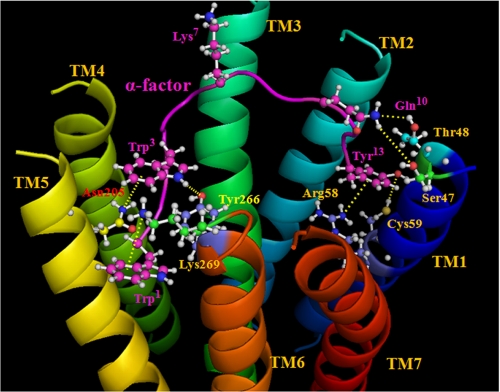
FIGURE 8.
Proposed model of the interactions of α-factor with residues of Ste2p. The TMs are labeled in different colors, and the α-factor backbone is shown as a purple ribbon. Selected residues of Ste2p and α-factor are shown in a ball-and-stick representation. All atoms in the selected α-factor residues are colored purple, except for nitrogen (blue) and oxygen (red), and atoms of Ste2p residues are in different colors to differentiate them from the α-factor atoms. The model shows the interactions of the N terminus and C terminus of α-factor with Ste2p TM5-TM6 and TM1, respectively. A bend in α-factor around the Pro8-Gly9, with the Lys7 side chain facing away from the TM domains, is shown. The Trp1 indole side chain of α-factor is proposed to form a cation-π (or hydrogen bonding) interaction with the Lys269 ϵ-amino group. Trp3 is in a hydrophobic pocket interacting with Asn205 (TM5) and Tyr266 (TM6). Gln10 of α-factor is suggested to be in close proximity to residues Ser47 and Thr48 (TM1). The Tyr13 side chain phenyl ring of α-factor is proposed to be involved in a cation-π interaction with the Arg58 (TM1) guanidinium moiety, and the phenolic OH (Tyr13) forms a hydrogen bond with the Cys59 (TM1) sulfhydryl group. All suggested interactions between α-factor residues and Ste2p residues are shown by the dashed lines in yellow.